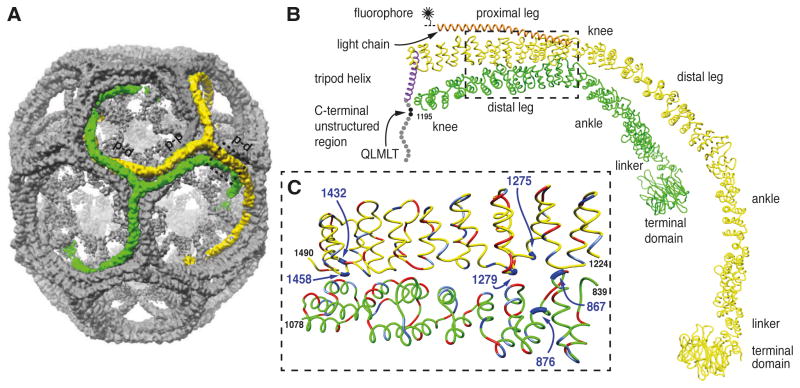
Figure 1. Location of histidine residues at the contacts between proximal and distal segments of neighboring triskelions.
A. Image reconstruction of a clathrin/AP-2 coat in the D6 barrel form (Fotin et al., 2004b). The contacts between parallel proximal and distal segments of the triskelions highlighted in green and yellow are indicated (p-d). The antiparallel proximal segments of the triskelions highlighted in green and yellow are indicated (p-p).
B. α-Carbon traces of single legs from neighboring triskelions (yellow and green, indicated by the dashed box in A) showing the invariant contact between parallel proximal and distal heavy chain segments viewed from the side. The heavy chain molecule shown in green comprises residues 1-1195 (includes part of the knee, distal leg, ankle, linker and terminal domain). The yellow heavy chain is associated with the central helix of its bound light chain (orange) and the α-helix of its trimerization domain forming part of the C-terminal tripod of the triskelion (purple). The disordered region of the heavy chain (residues 1630-1675) is indicated by the spheres with the binding site for Hsc70 (QLMLT motif) shown in black. The central helical segment of clathrin light chain is shown in orange. For imaging the light chain was labeled with a fluorophore at a single cysteine residue C-terminal to the helical segment. C. Close-up view of the interface between the proximal segment of the yellow heavy chain and the distal segment of the green heavy chain viewed from the side. The histidine residues mutated in this study are shown in dark blue and indicated by the arrows. Negatively charged residues are shown in red and positively charged residues are shown in light blue.