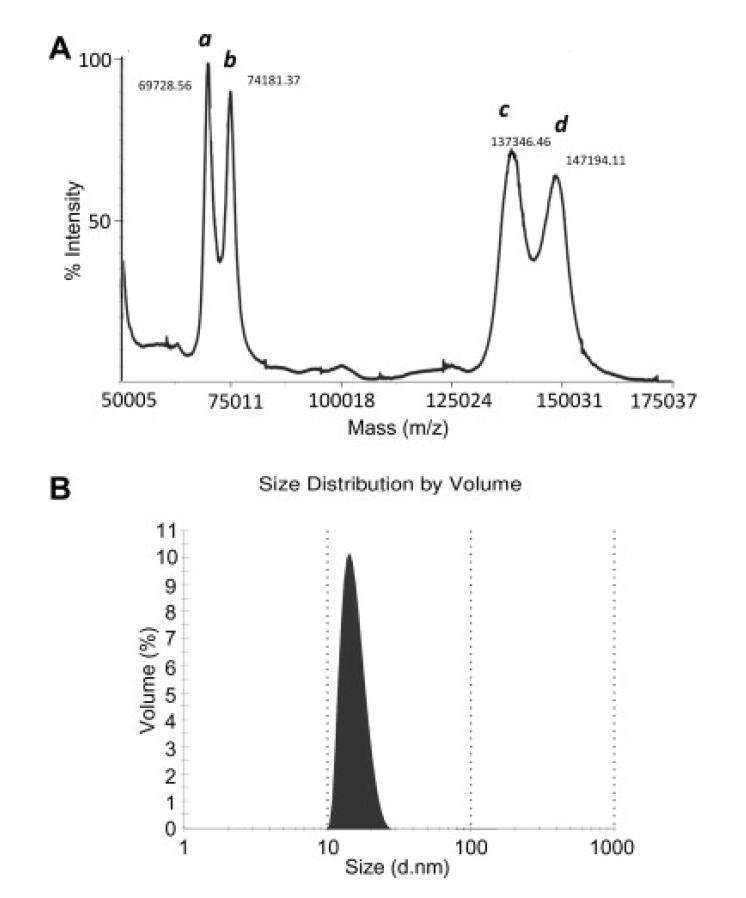
Figure 3.
Validation of rFH by mass spectrometry and dynamic light scattering. (A) The candidate recombinant FH (peaks a and c correspond to double-charged and single-charged species, respectively) and an internal standard (IgG1; peaks b and d correspond to double-charged and single-charged species, respectively) were analysed on a MALDI-ToF mass spectrometer. The experimentally derived mass of ~138,000 Da is in good agreement with the theoretical mass of rFH. (B) Dynamic light scattering was performed on rFH in PBS at a concentration of 1 mg/ml. No indication of aggregation was observed.