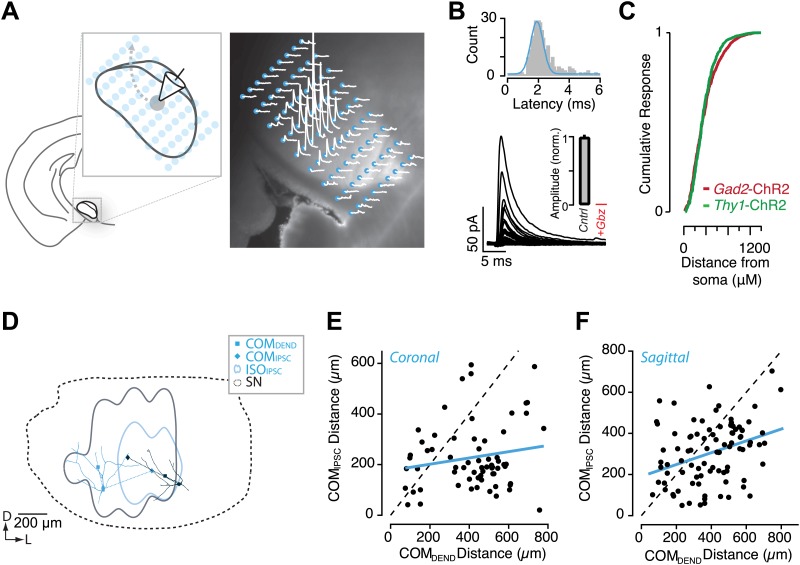
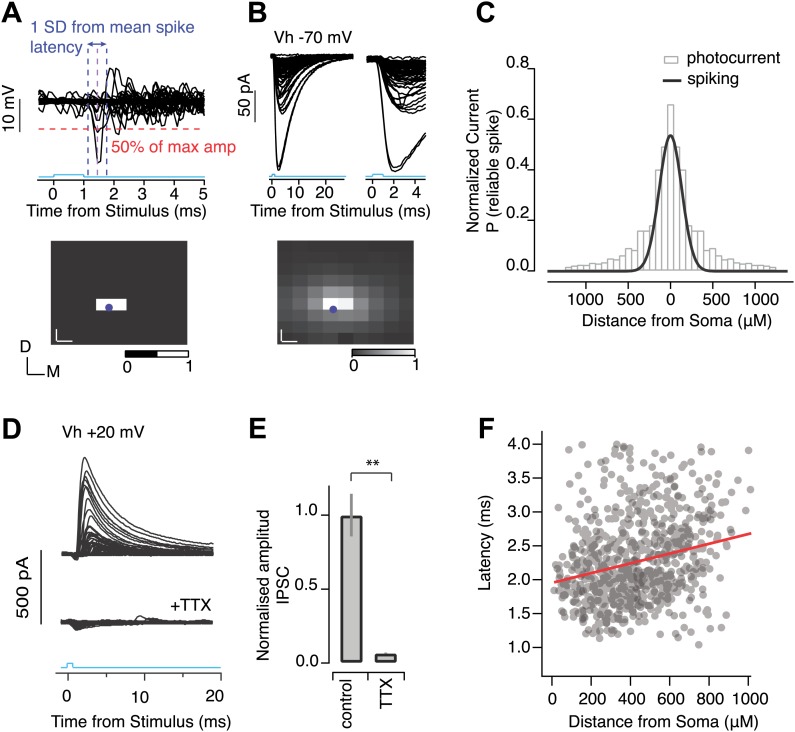
Figure 6. Circuit mapping of feedback inhibitory circuitry of SNr.
(A, left) Schematic of the experimental configuration used for channelrhodopsin-assisted circuit mapping. Whole-cell voltage clamp recordings were obtained from SNr GABA neurons while a focus laser beam was scanned across the SNr to excite SNr neurons with high spatial resolution. (A, right) Postsynaptic responses to individual photostimulations (white) were aligned to the DIC image of the slice. Stimulation points are indicated by cyan. (B) Example of evoked IPSCs from a single recording with a histogram of IPSC latencies for all recordings. Evoked IPSCs were completely inhibited in the presence of Gbz (B, insert; n = 8 cells; p<0.001, paired two tailed t test). (C) Cumulative histogram of response magnitude as a function of the distance between the stimulation site and recorded neuron in the Thy1-ChR2 (green) and Gad2-ChR2 (maroon) preparations. (D) Example of IPSC maps for two neurons. The dendritic arbor of each recorded neuron was reconstructed and transformed into the common SN reference frame (dotted line). For each neuron the center of mass (COM) of inhibition (COMIPSC, filled diamond), COM of dendritic field (COMDEND, filled square) and the isocontour of 50% inhibition (IS0IPSC, colored line) were calculated. (E and F) The COMIPSC was plotted as a function of the COMDEND for each neuron recorded in coronal (E; n = 14 cells) and sagittal (F; n = 16 cells) sections and the correlation fit estimated (blue line).