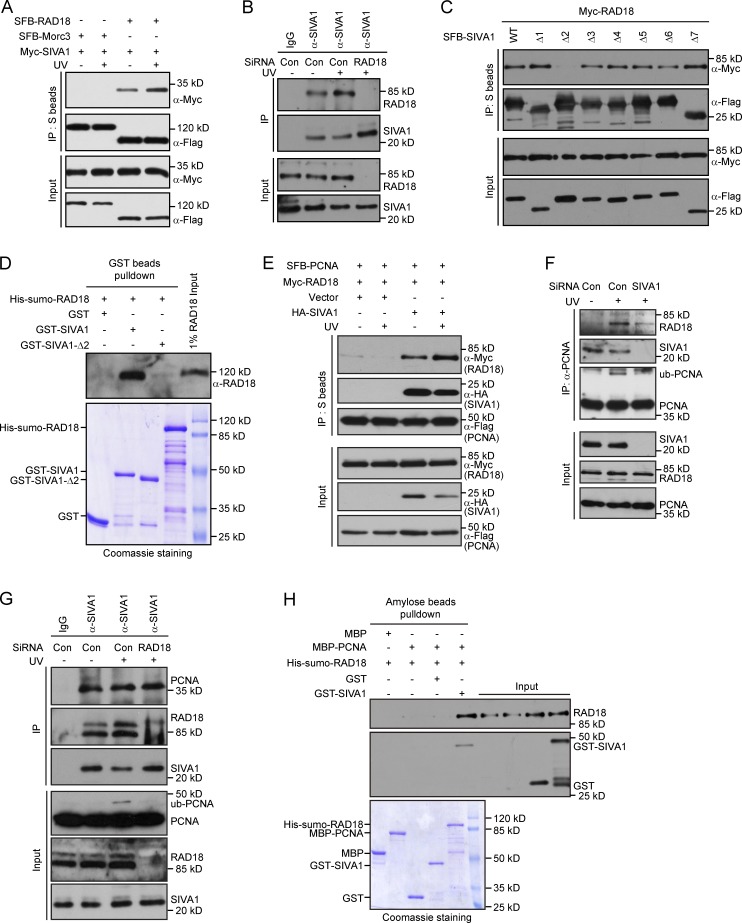
Figure 6.
SIVA1 associates with and recruits RAD18 to PCNA. (A) SIVA1 interacts with RAD18. HEK293T cells were transiently transfected with plasmids encoding SFB-tagged Morc3 or RAD18 together with plasmids encoding Myc-tagged SIVA1. 24 h after transfection, cells were left untreated or treated with 50 J/m2 UV for 1 h. Coprecipitation was performed using S protein beads, and immunoblotting was performed using antibodies as indicated. (B) Association of endogenous SIVA1 with RAD18 in HeLa cells was analyzed by immunoprecipitation using the anti-SIVA1 antibody. Cells transfected with the indicated siRNAs were treated with 50 J/m2 UV for 1 h or left untreated and then lysed with NETN buffer containing Benzonase. Cell lysates were incubated with protein A agarose beads conjugated with indicated antibodies, and Western blot analysis was performed as indicated. (C) Residues 40–58 of SIVA1 are responsible for RAD18 binding. HEK293T cells were transiently transfected with the indicated plasmids. 24 h after transfection, cells were treated with 50 J/m2 UV for 1 h. Coprecipitation was performed using S protein beads, and immunoblotting was performed using antibodies as indicated. (D) Direct in vitro binding between recombinant GST-SIVA1 and His-SUMO–RAD18 purified from E. coli. GST served as negative control for RAD18 binding. (top) RAD18 was detected by immunoblotting. (bottom) Purified proteins visualized by Coomassie staining. (E) Overexpression of SIVA1 enhances the interaction between RAD18 and PCNA. HEK293T cells were transiently transfected with the indicated plasmids. 24 h after transfection, cells were left untreated or treated with 50 J/m2 UV for 1 h. Coprecipitation was performed using S protein beads, and immunoblotting was performed using antibodies as indicated. (F) The RAD18–PCNA interaction is diminished in SIVA1-depleted cells. HeLa cells were transfected twice with control siRNA or siRNA specific for SIVA1. 48 h after transfection, cells were left untreated or treated with 50 J/m2 UV for 1 h. Cells were then Triton X-100 extracted and formaldehyde fixed, and cell lysates were immunoprecipitated with protein A agarose beads conjugated with anti-PCNA antibody. Western blot analysis was performed as indicated. (G) RAD18 depletion does not affect SIVA1–PCNA interaction. HeLa cells transfected with the indicated siRNAs were treated with 50 J/m2 UV for 1 h or left untreated and then lysed with NETN buffer containing Benzonase. Cell lysates were then incubated with protein A agarose beads conjugated with anti-SIVA1 antibody, and Western blot analysis was performed according to standard procedures. (H) SIVA1 facilitates RAD18–PCNA complex assembly in vitro. (top) RAD18 and SIVA1 were detected by immunoblotting. (bottom) Purified proteins were visualized by Coomassie staining. Con, control; IP, immunoprecipitation; ub, ubiquitin; WT, wild type.