FIG 1.
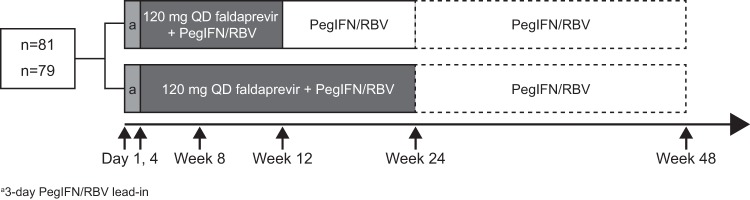
Study design. Treatment-naive patients were randomized to 12 or 24 weeks of faldaprevir plus pegylated interferon α-2a (PegIFN) and ribavirin (RBV), followed by PegIFN/RBV alone. Patients who achieved a maintained rapid virologic response (mRVR; HCV RNA below the lower limit of quantification [LLOQ; <25 IU/ml] at week 4 and undetected at weeks 8 and 12) stopped all treatment at week 24; those who did not achieve mRVR continued PegIFN/RBV to week 48. QD, once daily.
