FIG 5.
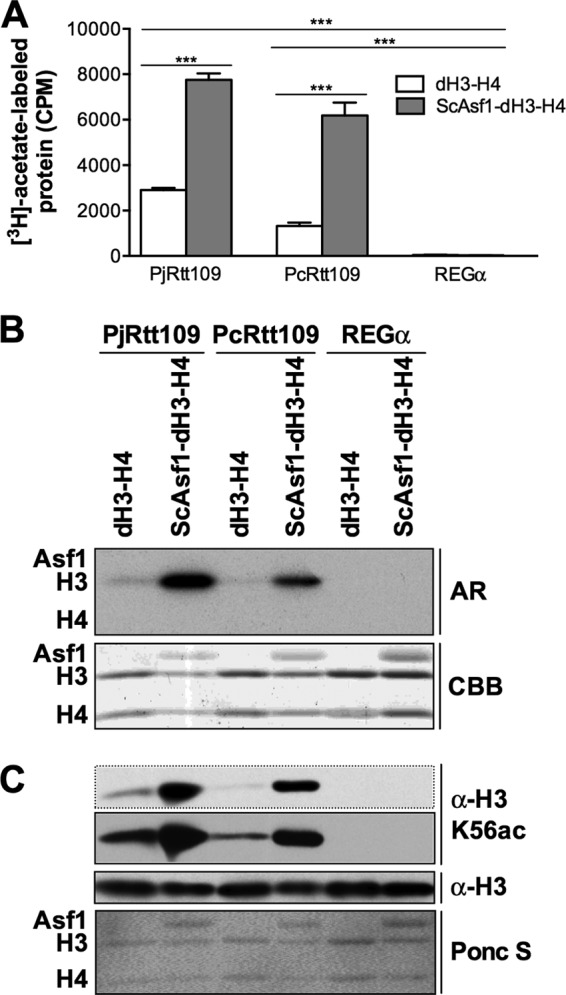
PjRtt109 HAT activity is enhanced by the histone chaperone Asf1 in vitro. (A) PjRtt109 HAT activity in vitro is enhanced by the addition of ScAsf1. ***, P < 0.001, pairwise comparisons between the dH3–H4 and ScAsf1-dH3–H4 substrates for each enzyme or pairwise comparisons with the REGα negative controls. Shown are representative results from a single experiment, with similar results being obtained in at least two other independent experiments. (B) Autoradiography of reaction mixture aliquots, as shown in panel A, demonstrates that acetylation is detected only on H3 and not on H4 or ScAsf1. Equal substrate histone contents were verified by CBB staining. (C) Western blotting of reaction mixture aliquots analogous to those shown in panel B versus H3K56ac, using nonradiolabeled acetyl-CoA as the substrate, was performed. Equal histone substrate contents were verified by Ponceau S staining and Western blotting.
