FIG 1.
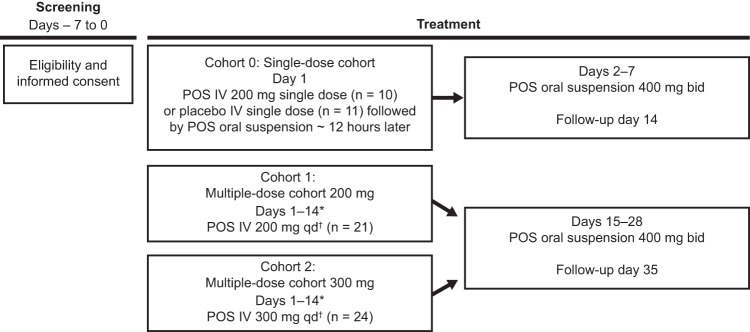
Study design, phase 1B. Each cohort was completed before subjects in the subsequent cohort were dosed. Safety was assessed throughout the study. *, PK samples for analysis of posaconazole were taken on days 1 and 14 at 0 h (predose), 1 h after start of infusion, immediately at the end of infusion, approximately 15 min after the end of infusion, and approximately 4, 8, 12, and 24 h after start of infusion. In cohorts 1 and 2, intravenous posaconazole (200 or 300 mg) was given twice daily as a loading dose on day 1. †, twice daily loading dose on day 1. bid, twice daily; IV, intravenous; POS, posaconazole; qd, once daily.
