Abstract
Macrophages take advantage of the antibacterial properties of copper ions in the killing of bacterial intruders. However, despite the importance of copper for innate immune functions, coordinated efforts to exploit copper ions for therapeutic interventions against bacterial infections are not yet in place. Here we report a novel high-throughput screening platform specifically developed for the discovery and characterization of compounds with copper-dependent antibacterial properties toward methicillin-resistant Staphylococcus aureus (MRSA). We detail how one of the identified compounds, glyoxal-bis(N4-methylthiosemicarbazone) (GTSM), exerts its potent strictly copper-dependent antibacterial properties on MRSA. Our data indicate that the activity of the GTSM-copper complex goes beyond the general antibacterial effects of accumulated copper ions and suggest that, in contrast to prevailing opinion, copper complexes can indeed exhibit species- and target-specific activities. Based on experimental evidence, we propose that copper ions impose structural changes upon binding to the otherwise inactive GTSM ligand and transfer antibacterial properties to the chelate. In turn, GTSM determines target specificity and utilizes a redox-sensitive release mechanism through which copper ions are deployed at or in close proximity to a putative target. According to our proof-of-concept screen, copper activation is not a rare event and even extends to already established drugs. Thus, copper-activated compounds could define a novel class of anti-MRSA agents that amplify copper-dependent innate immune functions of the host. To this end, we provide a blueprint for a high-throughput drug screening campaign which considers the antibacterial properties of copper ions at the host-pathogen interface.
INTRODUCTION
The continuous rise of drug-resistant or multidrug-resistant pathogenic bacteria has become a considerable challenge to the health care system and increasingly jeopardizes the earlier success of antibiotics as lifesaving treatments for bacterial diseases. In the United States, about 4.5% of all hospital patients develop an infection (1). About 99,000 patients that contract a nosocomial infection die each year as a result of these infections (1). According to the Centers for Disease Control and Prevention, the antibiotic resistance of bacteria in the United States costs an estimated $45 billion a year in excess health care costs (2). Accelerated efforts to identify new drugs that overcome bacterial drug resistance are therefore easily justifiable for humanitarian and economic reasons.
During the course of an infection, bacteria encounter a variety of host-derived antimicrobial agents. In particular, macrophages are known to expose microbes to excess copper and possibly zinc within their phagosomal compartments (3, 4) while other essential metal ions, such as iron and manganese, are being extracted (5). The identity of the phagosomal zinc transporter is still unknown, but the influx of copper is facilitated by ATP7A, a copper-specific transport protein which relocates from Golgi compartments to the phagosomal membrane (3). In vitro studies have shown that copper-sensitive Escherichia coli mutants are more susceptible to phagosomal killing than their respective wild-type strains (3). Likewise, copper homeostasis and resistance mechanisms are linked to virulence in many problematic pathogenic bacteria, including Listeria monocytogenes (6), Pseudomonas aeruginosa (7), and Mycobacterium tuberculosis (8, 9). In Staphylococcus aureus, copper stress leads to the repression of genes important for virulence and biofilm formation (10). CsoR has been identified as a crucial copper-dependent transcriptional regulator in S. aureus (11). The CsoR regulon includes the copAZ operon, which encodes a P1-type ATPase (CopA) and a copper chaperone (CopZ), and the copB mco operon, which is comprised of a multicopper oxidase (Mco) and another P1-type ATPase (CopB) (11).
The effects of elevated copper levels are multifaceted and include, for example, the destruction of iron-sulfur cluster proteins and metalloproteins, production of reactive oxygen species (ROS) by a Fenton-like chemistry, and interference with membrane integrity (12, 13). While these mechanisms are possibly the key to the antibacterial action of copper alloys, which are proven to restrict the spread of epidemic methicillin-resistant S. aureus (MRSA) in various health care settings (14, 15), the nonselective nature of these copper-dependent redox processes poses a challenge for target-directed applications in antibacterial therapy. However, the recent identification of copper-boosted compounds acting against M. tuberculosis and Neisseria gonorrhoeae at concentrations that are unlikely to affect intracellular copper levels (16, 17) contradicts these long-held models of activity. Indeed, other modes of action by which copper influences the antibacterial efficacy of compounds are now being investigated. For example, Manning et al. proposed a carrier function of copper ions for the antibiotic capreomycin (18), and select intracellular proteins have been identified as potential specific targets of some copper complexes. These targets include the isocitrate lyase from M. tuberculosis (19) as well as the succinate and NADH dehydrogenase of Neisseria gonorrhoeae (17).
In this study, we provide a road map for the high-throughput identification of copper-activated anti-MRSA compounds. Our primary screen identified several compounds with potent yet strictly copper-dependent anti-MRSA activities. We establish such molecules as a promising new class of anti-MRSA growth inhibitors potentially capable of enhancing host-induced copper-dependent innate immune functions.
MATERIALS AND METHODS
Bacterial strains, antibiotics, and compounds.
All S. aureus and MRSA isolates were obtained in a deidentified manner from UAB Laboratory Medicine. Bacteria were grown in Mueller-Hinton (MH) broth (Oxoid Ltd., Basingstoke, Hampshire, England). Thiocarlide, disulfiram, neocuproine, bathocuproine, EDTA, and copper sulfate were purchased from Sigma-Aldrich. The compounds glyoxal-bis(N4-methylthiosemicarbazone) (GTSM), pyruvaldehyde-bis(N4-methylthiosemicarbazone) (PTSM), and diacetyl-bis(N4-methylthiosemicarbazone) (ATSM) were synthesized as previously described (20, 21). Additional compounds were derived from a 50,000-compound in-house library (ChemBridge).
HTS assay and determination of MICs of select compounds.
All experiments were performed in 96-well plates using Mueller-Hinton broth as the growth medium unless otherwise stated. Compounds were tested at 10 μM in the presence of 50 μM copper in the high-throughput screening (HTS) assay. After preparing the compounds (16), S. aureus was added to achieve a final optical density at 600 nm (OD600) of ∼0.001 in a total volume of 160 μl. Plates were incubated on a Heidolph Titramax 1000 plate shaker at 300 rpm and 37°C for 7 to 8 h. The optical density as a quantitative surrogate marker of bacterial growth was then determined using a SynergyHT plate reader (BioTek). Background correction was performed against wells containing only medium. The activity of select compounds that decreased the growth of S. aureus by at least 90% (90% inhibitory concentration [IC90]) during the high-throughput screen was further analyzed in detailed dose-response curves. The assay conditions, incubation procedure, and analysis of growth inhibition were similar to those for the high-throughput screen.
PGFL assay.
In order to monitor intracellular fluctuations of copper, we used the fluorescent metal sensor Phen Green FL (PGFL; Molecular Probes). Bacteria were inoculated from an overnight culture and grown at 37°C until an OD600 of 1 to 2 was reached. Ten milliliters of that culture was centrifuged at 4,000 × g and washed 3 times in 10 mM HEPES buffer (pH 7.5). Aliquots of washed cells were treated for 1 h in HEPES buffer with 2.5 μM copper, GTSM, ATSM, CuIIGTSM, or CuIIATSM. Compounds and copper were removed by washing cells 3 times in HEPES buffer. Thereafter, cells were incubated at 37°C in HEPES buffer containing 2.5 μM Phen Green FL. Fluorescence was measured at 1 h posttreatment using a Synergy 2 plate reader (BioTek) equipped with a 460-nm excitation filter and a 520-nm emission filter. The Phen Green FL fluorescence is quenched by copper. Fluorescence intensity is therefore inversely correlated to the cell copper content.
Copper quantification analysis by ICPMS.
Cells were grown in MH medium, harvested by centrifugation, and resuspended in fresh MH medium to an optical density (OD600) of ∼2.5. Copper, ATSM, GTSM, EDTA, and the respective copper complexes were added to 30-ml cultures at the concentrations indicated below. Following incubation for 1 h at 37°C, samples were centrifuged and cell pellets were washed once with 500 μM EDTA in phosphate-buffered saline (PBS) and then twice with PBS. Washed and pelleted cells were subjected to acid digestion in 500 μl concentrated HNO3 (∼70%; Optima; Fisher Chemical) for 15 h at 65°C and thereafter diluted 20-fold with molecular biology-grade water and stored in certified metal-free tubes (Labcon). Elemental analysis was performed on a Thermo Element 2 HR inductively coupled plasma mass spectrometer (ICPMS; Thermo Fisher Scientific, Bremen, Germany) equipped with an electrospray ionization autosampler (Elemental Scientific, Omaha, NE). The diluted acid-digested samples were taken up by self-aspiration via a 0.50-mm (inner diameter) sample probe and sample capillary to a nebulizer and spray chamber. The copper content was determined on the basis of the amount of the 63Cu isotope at medium resolution (R = 4,300). Samples were analyzed in triplicate, and error bars represent standard deviations.
Structure prediction and visualization.
The chemical structures of GTSM, ATSM, and their respective copper complexes were drawn and edited using Marvin Sketch software (ChemAxon LLC). The low-energy conformers were generated and edited in Marvin Space software (ChemAxon LLC) and are displayed in van der Waals surface mode.
RESULTS
A high-throughput drug screening assay for copper-activated anti-MRSA drugs.
Methicillin-susceptible S. aureus (MSSA) infections cause significant health care problems. Methicillin-resistant S. aureus (MRSA) has added to this challenge, and novel drugs with activities against MRSA are clearly needed. This research started as a knowledge transfer effort from our recent finding that copper complexation could vastly enhance the antibacterial activity of certain compounds against M. tuberculosis (16). To test whether this concept was also applicable to MRSA, we developed a high-throughput drug screening assay allowing us to search for compounds that synergistically enhance the antibacterial properties of copper ions against S. aureus.
Our approach is based on the simultaneous screening of a compound library for antibacterial activities under trace copper and copper-supplemented culture conditions (16). Only compounds that would be inactive in trace copper medium but active when copper ions are supplemented are considered potential hits. For this purpose, we identified Mueller-Hinton (MH) medium to be a suitable growth medium. Its pH remains relatively constant upon the addition of up to 1 mM CuSO4, and its composition minimizes the formation of undesirable insoluble inorganic copper complexes (22). At the same time, this medium efficiently supports the growth of a wide range of bacteria, enabling the comparison of drug effects between different Gram-positive and Gram-negative pathogens.
We used the optical density (OD600) as a quantitative readout and surrogate marker of bacterial growth. This strategy made the assay (i) inexpensive, as no additional growth indicator dyes, such as alamarBlue, were needed and (ii) less prone to manipulation-induced variations due to fewer handling steps. To ensure that no laboratory-induced adaptive mutations in S. aureus would affect the screen (23), we chose to work invariably with methicillin-susceptible and methicillin-resistant clinical isolates of S. aureus.
To define the copper-supplemented condition for the actual screen, we initially determined the level of tolerance of MSSA and MRSA to copper in MH broth. We found that both strains, under the chosen assay conditions, grew normally in the presence of copper up to a concentration of at least 300 μM (data not shown). Although macrophages can challenge bacteria with a copper concentration of up to 400 μM (24), we chose to screen at a copper concentration of 50 μM, which is about twice the copper concentration present in human blood (25, 26). We reasoned that a slightly higher copper concentration in the assay may increase its sensitivity, thereby aiding in the detection of weaker synergistic interactions between copper and compounds which could be useful for subsequent structure-activity relationship analysis. Under copper-supplemented and trace copper conditions, MSSA entered into stationary growth within 7 to 8 h, at which time the OD600 readings were taken.
The quality and performance of the assay were tested by the use of Z′-tests. The Z′-factor that was calculated from these results is a statistical value designed to reflect the dynamic range of the assay, as well as the variation associated with the signal measurements. As the Z′-factor is dimensionless, it can be used for assay optimization purposes. An assay with a Z′-factor of 1.0 would represent an ideal assay. To be considered for HTS, an assay should be characterized by a Z′-factor of >0.5 (27). Using neocuproine, a well-defined copper-complexing compound and cell growth inhibitor (16, 28), the Z′-factor for the MSSA assay in a 96-well plate format was >0.8 (see Fig. S1A in the supplemental material), indicating that the system is highly reliable and reproducible and, thus, is suitable for HTS.
Proof-of-concept drug screen.
To transfer the assay to a robotic platform, we performed a small proof-of-concept screen using a compound library that was enriched for rationally selected copper-chelating compounds. The goal of this effort was to demonstrate that the assay is capable of identifying copper-boosting compounds with anti-MRSA activity and to detail the potency of these compounds by titrating relevant hit molecules in the presence or absence of copper on MSSA and MRSA.
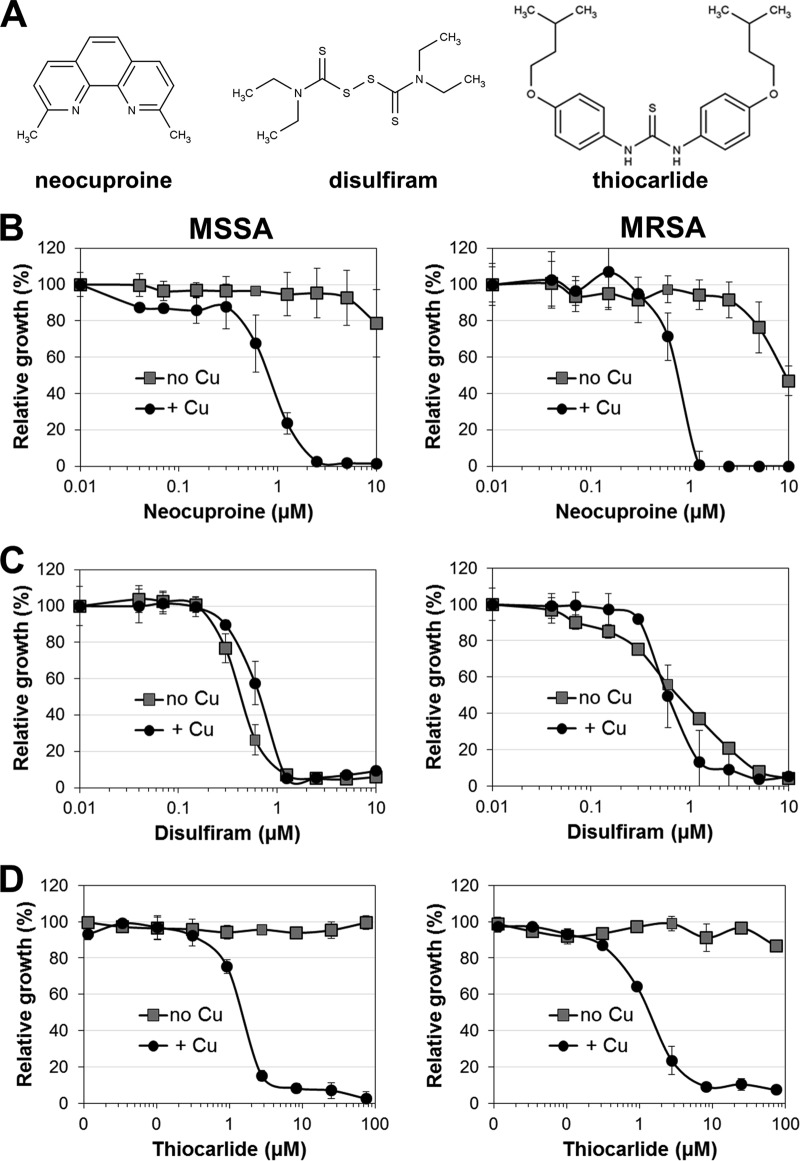
The well-characterized toxic copper chelator neocuproine (Fig. 1A) showed limited antibacterial activity at high concentrations in trace copper medium but was highly effective under copper-activated conditions (Fig. 1B). The low-level activity of neocuproine in trace copper MH broth is likely attributable to the ability of neocuproine to chelate even trace amounts of copper or to deplete metabolically essential intracellular copper reservoirs. The membrane-impermeant analogue bathocuproine lacked antibacterial properties under either condition (data not shown), indicating that the neocuproine-copper complex exerts its activity intracellularly.
FIG 1.
Identification of copper-dependent anti-MRSA compounds. The random compound library used for a proof-of-concept screen was spiked with several compounds with reported potential copper-complexing ability, some of which were found to be antibacterial compounds. (A) Molecular structures of neocuproine, disulfiram, and thiocarlide. (B to D) The antibacterial activities of neocuproine (B), disulfiram (C), and thiocarlide (D) were plotted over the respective drug concentration for trace copper (no Cu) and copper-supplemented (+ Cu) conditions. (Left) Experiments performed using a clinical MSSA strain; (right) data obtained using a clinical MRSA strain. All experiments were performed in triplicate, and the means ± standard deviations are presented for each drug concentration. Data are representative of those from at least three independent experiments.
Previous studies have indicated that the antialcoholism drug disulfiram (Fig. 1A) inhibits the growth of MRSA (29, 30). Disulfiram and its metabolites form copper complexes in the stomach, the small intestine, and the blood (31). Some investigators proposed that copper complexation may contribute to disulfiram's biological activity (30). However, in our assays the activity of disulfiram against MSSA and MRSA in MH medium appeared to be copper independent. Titration curves indicated an IC90 of 1.25 μM (0.38 μg/ml) (Fig. 1C) under trace copper and copper-supplemented assay conditions. The previously reported inhibitory concentration of disulfiram on MRSA was 4.4 μM (1.33 μg/ml) (29). Although a copper-dependent mechanism of disulfiram is not obvious, we cannot exclude the possibility that trace copper in MH medium (22) may be sufficient to form disulfiram-derived copper complexes. Lot-to-lot variations of the trace metal content in MH medium (32, 33) may then explain the higher potency of disulfiram in our study. Nevertheless, our assay confirms that disulfiram is indeed effective against MRSA. Reported potential cellular targets of disulfiram and other dithiocarbamates in bacteria include beta-carbonic anhydrases of M. tuberculosis (34) and the betaine aldehyde dehydrogenase of Pseudomonas aeruginosa (35). Specific target sites in S. aureus have not yet been reported. However, its genome does encode a betaine aldehyde dehydrogenase (betB) (36), which could be a possible target of disulfiram.
As a striking example that our approach is capable of revealing previously unnoticed anti-S. aureus activities, thiocarlide (Fig. 1A), an old antitubercular thiourea drug (37), was identified to exert copper-dependent anti-MRSA activity with an IC90 of 5 μM (2 μg/ml) (Fig. 1D), which is 100-fold more potent than the inhibitory concentrations from previously published assays where MH medium was also used (38). This finding suggests that even approved and characterized drugs should potentially be revisited for antibacterial MRSA activity if they were once found to be inactive on the basis of in vitro assays and have not been tested in in vivo models.
GTSM exerts potent activity against MSSA and MRSA.
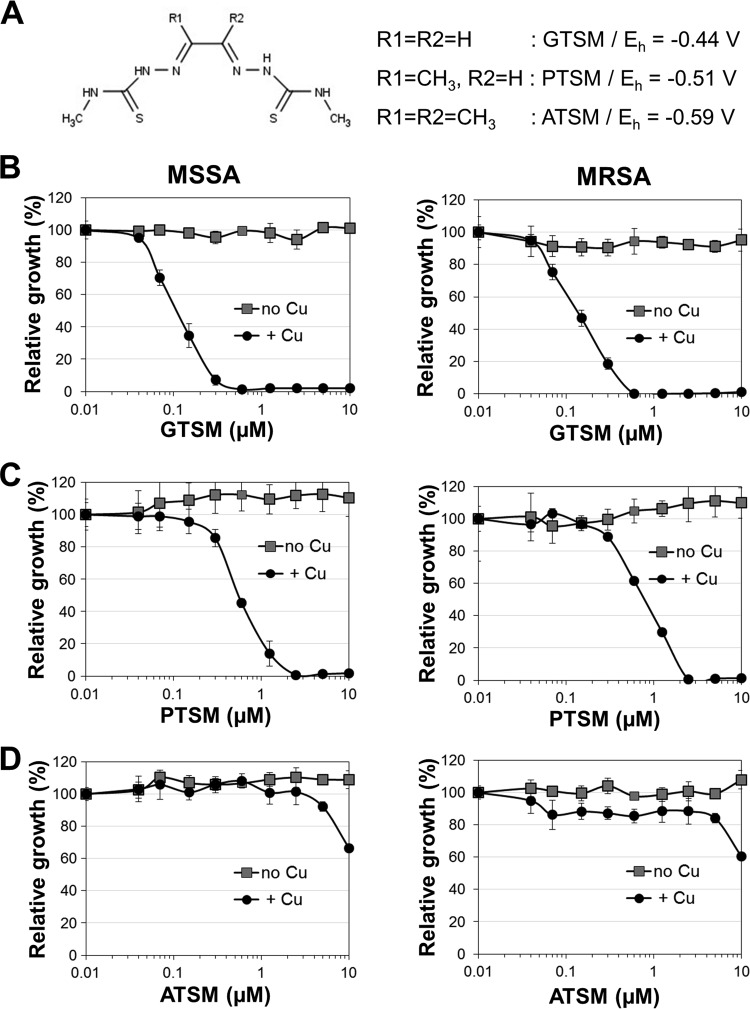
The screen further identified glyoxal-bis(N4-methylthiosemicarbazone) (GTSM) to be an anti-S. aureus compound active against MSSA and MRSA (Fig. 2 and 3A and B). In contrast to the activity profile of GTSM in M. tuberculosis (16) and Neisseria gonorrhoeae (17), no copper-independent activity against S. aureus was detected (Fig. 3B). Even at 10 μM, GTSM had no significant anti-S. aureus activity in trace copper medium. In the presence of copper, however, GTSM was extremely potent and exhibited an IC90 of 0.3 to 0.6 μM on all tested clinical isolates (Fig. 2A and B). These results indicate that GTSM is able to evade the preexisting drug resistance mechanisms of MRSA strains and is acting by a novel mode of action.
FIG 2.
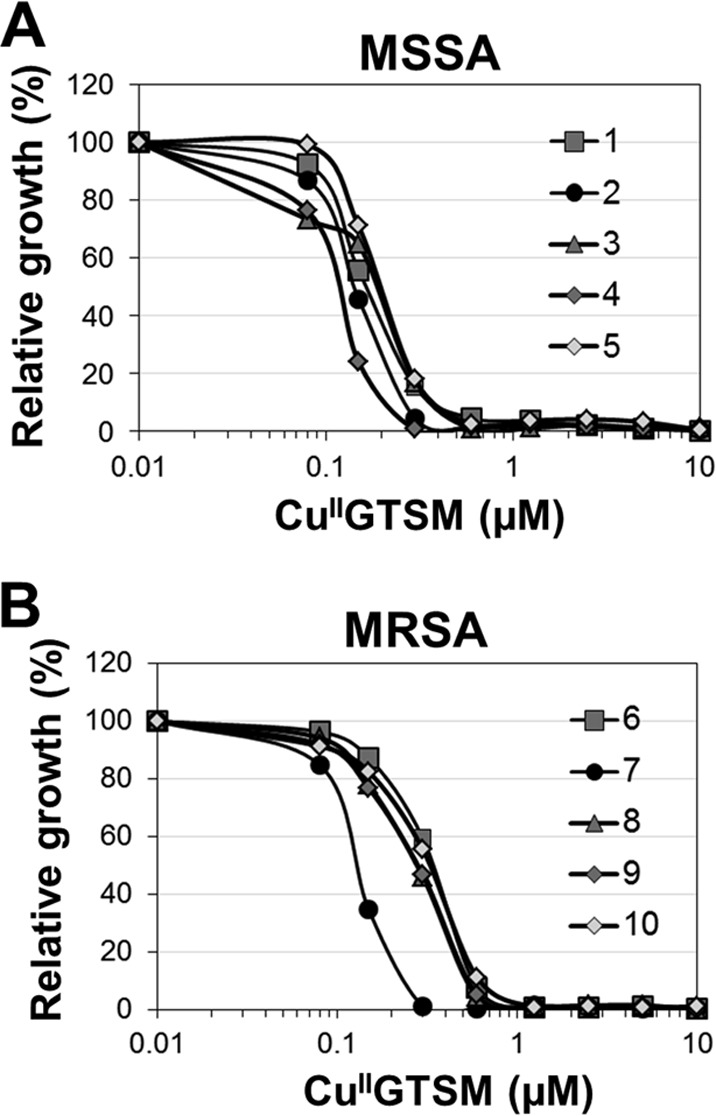
Activity of GTSM against S. aureus. To confirm that the copper-dependent antibacterial effect of GTSM was not limited to a single isolate, we titrated GTSM in the presence of 10 μM copper on five MSSA isolates (isolates 1 to 5) (A) and five MRSA isolates (isolates 6 to 10) (B). Data are presented as growth normalized to that of untreated wells. Shown is a representative data set out of three independent experiments.
FIG 3.
Correlation between redox potential and antibacterial properties of bis(thiosemicarbazone). (A) Molecular structures and redox potentials (Eh) of GTSM-, PTSM-, and ATSM-copper complexes. Redox potentials were taken from reference 43. (B to D) Antibacterial effects of GTSM (B), PTSM (C), and ATSM (D) on MSSA (left) or MRSA (right) in copper-supplemented (+ Cu) and trace copper (no Cu) MH medium. All experiments were performed in triplicate, and the mean values ± standard deviations of the drug effect on cell growth are presented for each compound concentration tested. Data are representative of those from at least three independent experiments.
The susceptibility of bacteria to antibiotics and copper can vary in response to the utilized growth medium (39, 40). To ensure that the copper-dependent antibacterial properties of GTSM are not a medium-specific artifact, we confirmed its activity in an entirely different medium. Unlike MH medium, RPMI 1640 consists exclusively of defined components, such as inorganic salts, vitamins, amino acids, and d-glucose as the carbon source. Important for our purpose, this medium does not contain any documented copper source. Using RPMI 1640, we established the same drug screening assay with trace copper and copper-supplemented screening conditions. Cell growth was found to be slightly delayed relative to that in MH medium, but the RPMI 1640-based Z′-test revealed a similar Z′-factor of >0.7 (see Fig. S1B in the supplemental material). Also, the copper-dependent anti-MRSA effect of GTSM could be fully reproduced (see Fig. S1C in the supplemental material). As RPMI 1640 does not contain significant amounts of zinc or iron, the medium may also be ideal to test possible compound interactions with other metal ions. For example, silver ions were also reported to enhance the efficacy of certain antibiotics (41). MH broth would be less suitable for this purpose due to its varying metal content and its undefined ingredients (32). However, while RPMI 1640 is suitable to identify synergistic interactions between copper ions and possibly other physiologically relevant divalent metal ions, it is important to appreciate that standard RPMI 1640 medium does not lend itself to be a long-term growth medium. Precultures for the actual screening assay need to be maintained in MH broth or any other medium that optimally supports the normal growth of S. aureus.
Selective antibacterial properties of GTSM.
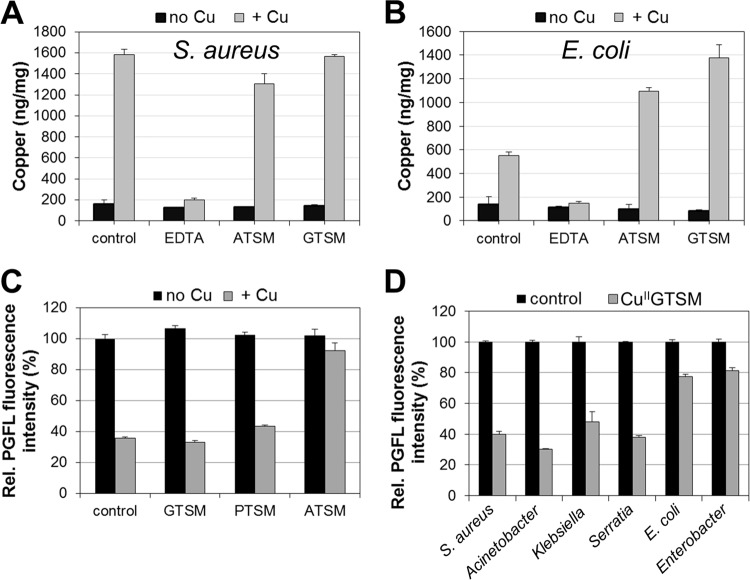
We previously described GTSM to be a copper-dependent anti-M. tuberculosis candidate compound (16), and in this study, we found it to be highly potent against S. aureus. Therefore, we wanted to know whether GTSM is broadly active against other bacterial pathogens. For this purpose, we screened a panel of 50 different clinically isolated Gram-positive and Gram-negative bacterial pathogens for susceptibility to CuIIGTSM. In this screen, the sole identified susceptible bacterium was S. aureus. Gram-negative bacteria, including various clinical isolates of Acinetobacter baumannii, Klebsiella pneumoniae, Serratia marcescens, Escherichia coli, Enterococcus faecium, Stenotrophomonas maltophilia, and P. aeruginosa, were not susceptible to GTSM (Fig. 4) in trace copper or in copper-supplemented MH broth. These data indicate that GTSM could exert its antibacterial activity on S. aureus and M. tuberculosis by inflicting damage to pathogen-specific targets.
FIG 4.
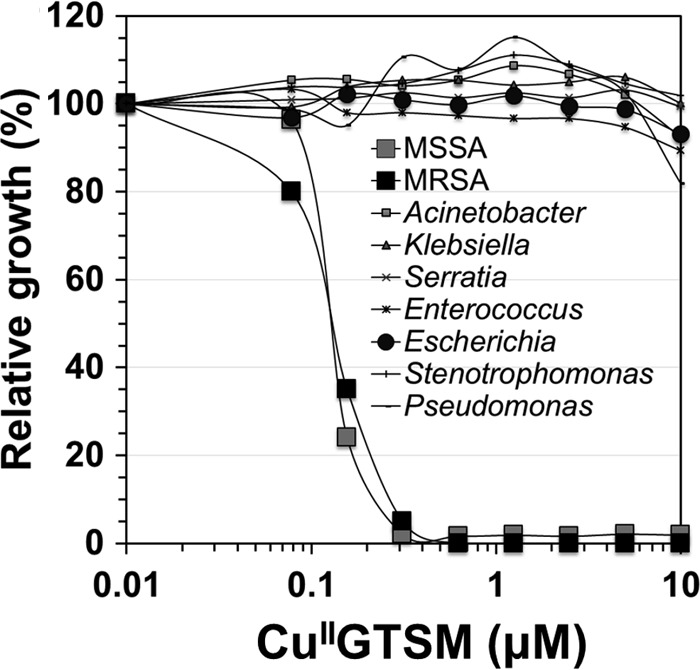
GTSM is specifically active on Staphylococcus aureus. To assess whether GTSM would eventually be broadly usable as an antibacterial agent, we tested its copper-dependent antibacterial activity on several clinically isolated bacterial species belonging to 7 genera. The antibacterial effect was plotted as growth normalized to cell growth in untreated control wells. The bacterial genera mentioned in the figure refer to the following bacterial species: MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; Acinetobacter, A. baumannii; Klebsiella, K. pneumoniae; Serratia, S. marcescens; Enterococcus, E. faecium; Escherichia, Escherichia coli; Stenotrophomonas, S. maltophilia; Pseudomonas, P. aeruginosa.
The anti-S. aureus activity of bis(thiosemicarbazone)-copper complexes is linked to their redox potential.
The bioactivities of clinically investigated bis(thiosemicarbazone) ligands have been linked to their redox stability. In particular, the reduction of CuII to CuI is thought to destabilize the complex, leading to the release of copper inside cells (42). This process is more likely to occur in complexes with a high redox potential (42, 43). To investigate whether the anti-S. aureus properties of CuIIGTSM may be attributable to reductive deterioration and the subsequent release of copper ions from the complex into the cytoplasm, the potency of three closely related bis(thiosemicarbazone) ligands (GTSM, PTSM, ATSM) with different redox potentials (Eh; −0.43, −0.51, and −0.59, respectively [43]) were compared (Fig. 3A). Indeed, the compound with the highest redox potential, CuIIGTSM, exerted the highest potency toward S. aureus (IC90 = 0.3 μM) (Fig. 3B), while ATSM, the compound with the lowest redox potential, was completely inactive (Fig. 3D). Accordingly, CuIIPTSM, which has an intermediate redox potential, still had some activity (IC90 = 1.25 μM) (Fig. 3C). A similar trend in the decline in potency between GTSM and ATSM was previously observed in M. tuberculosis and N. gonorrhoeae, even though CuIIATSM was active in these systems at 2.5 and 0.1 μM, respectively (16, 17).
Quantitative analysis of Cu by ICPMS confirmed that, despite their contrasting activities, both compounds are taken up by S. aureus in almost identical amounts, as indicated by the 8- to 10-fold higher cell copper levels of CuIIATSM- or CuIIGTSM-treated cells (Fig. 5A). However, these cell copper levels are similar to the copper contents of cells treated with only copper, indicating that the activity of CuIIGTSM is not due to a cellular copper overload, as has been proposed for other copper-coordinating compounds (44). Interestingly, in E. coli, which is resistant to GTSM, treatment with either compound led to a 2- to 2.5-fold higher cellular copper content than treatment with copper alone (Fig. 5B). Hence, the cell copper content is not indicative of the antibacterial properties of these compounds.
FIG 5.
Effect of bis(thiosemicarbazone)-copper complexes on cellular copper levels. (A and B) ICPMS analysis of S. aureus (A) and E. coli (B). Controls indicate treatment with (gray bar) or without (black bar) 10 μM copper. EDTA, GTSM, and ATSM (black bars) and the respective copper complexes (gray bars) were provided at a concentration of 10 μM. (C) S. aureus was treated with either free (black bars) or copper-loaded (gray bars) bis(thiosemicarbazone) ligands. The fluorescence intensity of Phen Green FL (PGFL) is inversely correlated to the cellular copper content. Cells derived from the plain (black bar) or copper-supplemented (gray bar) condition are referred to as controls. (D) Phen green FL analysis of various bacterial species in response to GTSM (black bars) or CuIIGTSM (gray bars). All agents for which the results are provided in panels C and D were provided at a concentration of 2.5 μM.
In order to determine whether complexes with a high redox potential destabilize inside the cells and release copper, we used the fluorescent copper sensor Phen Green FL (PGFL) (45). In S. aureus, copper quenches the fluorescence of the activated PGFL sensor in a concentration-dependent manner (see Fig. S2 in the supplemental material). The sensor detected increasing copper levels in cells exposed to 1.5 to 12.5 μM extracellular copper. ICPMS analysis confirmed that the cell copper content of S. aureus is indeed a function of the extracellular copper content (see Fig. S3A in the supplemental material), which was in contrast to the findings for E. coli, where cell-associated copper levels were independent of the extracellular copper concentration (see Fig. S3B in the supplemental material). It should also be noted that, in our system, S. aureus tolerates copper at concentrations of up to 400 μM without significant suppression of growth (data not shown). Treatment with 2.5 μM copper decreased the PGFL fluorescence by ∼60% (Fig. 5C). Similar quenching rates were obtained from CuIIGTSM- or CuIIPTSM-treated samples (Fig. 5C). In contrast, fluorescence quenching by CuIIATSM was only minor (Fig. 5C). Since ATSM and GTSM equally increased the cell copper content, as determined by ICPMS (Fig. 5A), the observed differential in fluorescence quenching may thus support a redox-dependent copper release mechanism for these compounds. In contrast, treatment of E. coli and Enterococcus faecium, which are both resistant to CuIIGTSM, generated only a minor decrease in fluorescence (Fig. 5D). However, in other GTSM-resistant bacteria, including Acinetobacter baumannii, Klebsiella pneumoniae, and Serratia marcescens, fluorescence quenching was observed, despite their resistance to the compound (Fig. 5D), suggesting that these organisms counteract CuIIGTSM toxicity by means different from those used by E. coli or Enterococcus faecium.
Copper coordination changes the conformation of bis(thiosemicarbazone) ligands.
As the copper-free GTSM ligand is not active, we investigated potential mechanisms by which copper could activate the complex. The predicted low energy conformation and van der Waals surface model revealed remarkable structural differences between the free GTSM ligand and its respective copper complex (Fig. 6A and B). While the GTSM molecule is linear with a dumbbell-like appearance (Fig. 6A), CuIIGTSM is more compact with the ligand wrapped around the copper ion (Fig. 6B). It is therefore conceivable that potential binding sites on target proteins accommodate only the metal complex and not the free ligand. In extension, structural differences between ATSM and GTSM (Fig. 6C) could considerably contribute to the variable anti-S. aureus activities of those copper complexes. GTSM and ATSM are tetradentate ligands that interact with a variety of metal ions through their N2S2 donor motif (46). However, other physiologically relevant metal ions, including CoII, ZnII, MnII, and FeIII, if coordinated to GTSM, did not activate the complex (see Fig. S4 in the supplemental material), despite presumably similar structures. While these results are not conclusive in regard to the importance of intramolecular structural rearrangements for the observed activity, they conclusively demonstrate that the copper ion is absolutely essential for the growth-inhibitory activity of the CuIIGTSM complex.
FIG 6.
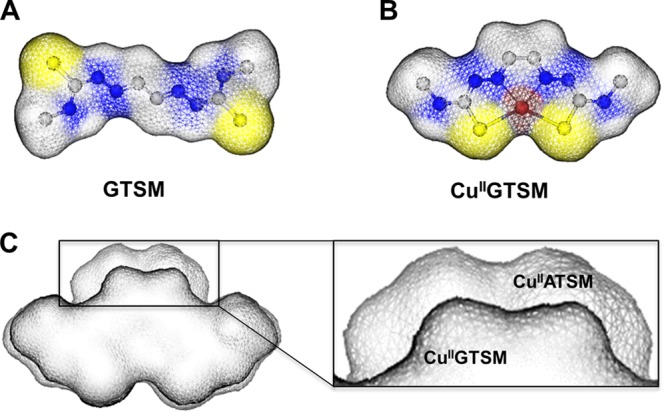
Effect of copper coordination on the molecular conformation of bis(thiosemicarbazone) ligands. Shown are van der Waals surface representations of GTSM (A) and CuIIGTSM (B). (C) Overlay and alignment of the surface models of CuIIATSM (back) and CuIIGTSM (front). The area of misalignment is magnified in the box to the right. Yellow, blue, gray, and red indicate sulfur, nitrogen, carbon, and copper atoms, respectively. Hydrogen atoms are not shown.
DISCUSSION
Copper ions have essential metabolic functions in most cells but are also crucial players at the host-pathogen interface. It is well documented that bacteria encounter elevated copper levels in various locations and subcellular environments within the host (8, 24, 47), which suggests that drugs are also subjected to copper exposure at these naturally occurring copper reservoirs. Thus, the emerging importance of copper ions for innate immune functions (12, 48, 49) provides a physiological rationale to consider the medium copper content during drug screens.
Most utilized growth media are not well standardized in regard to their copper content. While some investigators use minimal medium, others prefer media whose compositions are enriched with a variety of mostly chemically undefined supplements. Not surprisingly, results obtained from different drug screens, even those using compound libraries from the same commercial source, are sometimes hardly comparable. Here we provide the proper screening tools and demonstrate that standardization of the copper content in the growth medium can optimize the process of antibacterial compound discovery. This was achieved by the development of an HTS assay for anti-MRSA compounds, which was then used to screen a small compound library for activity against S. aureus in MH medium under both copper-supplemented and non-copper-supplemented conditions. For the first time, this strategy revealed the copper-dependent antibacterial properties of the antibiotic thiocarlide against MRSA (Fig. 1D). Thiocarlide was previously found to be inactive against S. aureus (38).
The limited screen further identified the bis(thiosemicarbazone) GTSM to exert anti-MRSA activity as a function of the medium copper content. GTSM has shown some promise in mouse models of Alzheimer's disease (50). We expanded our analysis of bis(thiosemicarbazone) and included ATSM and PTSM, which have previously been administered to mice at doses between 2.5 and 100 mg/kg of body weight/day (50, 51). Both compounds have been clinically investigated as tracer agents for tumor imaging (21, 43) and as part of anticancer regimens (52), demonstrating that such copper-boosted drugs are physiologically tolerated. Also, the effects and applications of bis(thiosemicarbazone) can be specific. Most recently, Cater et al. demonstrated that even highly related bis(thiosemicarbazone) compounds possess distinctive biofunctions, with GTSM being active against prostate cancer cells and ATSM being inactive (51). This is interesting, as GTSM was also found to be more potent than ATSM against M. tuberculosis and N. gonorrhoeae (16, 17). It is also important to appreciate that the antibacterial activity of GTSM and its copper complex is limited to only a few bacterial species. While we tested a total of 50 clinical isolates that represent at least 8 different species, activity was restricted to S. aureus (Fig. 4). Other susceptible species include M. tuberculosis (16) and N. gonorrhoeae (17).
While the drug-screening design presented here is cognizant of the possibility that drugs need to interact with copper to exert an antibacterial effect, the drug screen does not select for a specific mechanism of action. Compounds might induce a copper susceptibility phenotype by inhibiting a crucial component of the bacterial copper homeostasis system rather than interacting directly with copper. This has been described for the copper homeostasis protein multicopper oxidase (CueO), which is inhibited by silver ions (53). Copper can also function as a carrier to increase the uptake of compounds across membranes, as proposed by Manning et al. (18), but the exact opposite scenario in which the compounds serve as the carrier for copper ions is also conceivable and exemplified by the potential catechol-mediated uptake of copper in E. coli (54). It is also known that the coordination of copper with a ligand can change its mode of interaction with bacterial membranes, affecting its bioactivity and bioavailability, as proposed for moxifloxacin (55). Other factors that determine the biofunctions of copper complexes may include the Fenton-like chemistry of copper and certain aspects of its coordination and redox chemistry. Given such diversity, the precise nature of copper-enhanced antibacterial compounds needs to be carefully determined and should not be generalized.
Copper accumulation inside the bacterial cell is one of the leading arguments to explain the antibacterial properties of copper complexes, and in some cases it holds true (54). However, it should be noted that copper-susceptible E. coli and Mycobacterium smegmatis mutants can accumulate a lot more copper than their respective wild types in copper-enriched medium and are still viable (8, 54). Thus, the growth-inhibitory effects of copper complexes may not necessarily be the result of copper overload. Indeed, CuIIGTSM did not enhance the copper accumulation of S. aureus beyond the levels achieved by treatment with only copper (Fig. 5A). This finding is in excellent agreement with the findings for the N. gonorrhoeae system (17). Copper overload is therefore not indicated to be a potential mode of action for CuIIGTSM.
As we point out, the formation of a complex between copper ions and the polydentate ligand GTSM substantially changes the conformation of the ligand (Fig. 6A and B). Such structural rearrangements may enable an exclusive interaction between the copper complexes and putative binding sites on target proteins. In such a scenario, copper serves as an intramolecular adhesive. However, GTSM complexes with other metal ions that have nearly identical structural configurations were not active on S. aureus, highlighting that the copper ion is an indispensable structural and functional feature. Copper complexes are known to have artificial nuclease-, metalloprotease-, or superoxide dismutase-like activities (56). Unfortunately, none of these activities can safely discriminate between host and bacterial cells, which is why copper complexes were primarily developed into analytical tools rather than antibacterial agents. However, this long-standing paradigm is about to change. The recent work by Djoko and coworkers suggests that CuIIGTSM and CuIIATSM specifically interact with the NADH dehydrogenase of N. gonorrhoeae (17), delivering proof of concept that copper complexes can be developed for target-specific applications at the host-pathogen interface. Their data on N. gonorrhoeae (17) and our data on M. tuberculosis (16) and S. aureus support the following mode of antibacterial action for CuIIGTSM: the redox-dependent separation of copper from its complex with GTSM is of potential relevance for the activity of CuIIGTSM, which was indicated by the Phen Green FL assay (Fig. 5C) and the inverse correlation between the potency and redox stability of bis(thiosemicarbazone) complexes (Fig. 3). While this may not overwhelm cellular copper homeostasis systems in general (Fig. 5A), docking of the complex to a specific protein target and the subsequent reduction of CuII to CuI may lead to a local increase of redox-active copper in close proximity to sensitive enzymatic functions. For instance, CuIIGTSM may be reduced and destabilized by the action of respiratory NADH dehydrogenase, as proposed by Djoko et al. (17). The copper ions could then inflict damage through adverse interactions with critical thiols or iron sulfur clusters of adjacent respiratory or metabolic proteins, as reported elsewhere (13, 57, 58).
In summary, our data stress the importance of considering copper availability in the medium for antibacterial drug screens. To this end, the drug screening assay presented here could serve as a blueprint on how to improve current anti-MRSA drug screens toward the efficient discovery of novel copper-activated anti-MRSA drugs. While the importance of metal ions in presynthesized complexes for anticancer therapy is undisputed (59, 60), endogenous metal ions do not seem to have attracted much appreciation as potential determinants of desirable pharmacological activities of drugs. As microbial adaptation to antibiotics already occurs at a faster pace than the discovery and development of new drugs, the incorporation of in vivo transition metal coordination chemistry in the design of whole-cell-based in vitro drug discovery approaches may thus create a novel opportunity to prevail over health care-related microbial drug resistance.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by NIH grant RO1 AI104952 to F.W., NSF grant 1242765 to S.H.B., and the University of Alabama at Birmingham Center for AIDS Research (UAB CFAR), an NIH-funded program (P30AI027767-24) that was made possible by NIAID, NIMH, NIDA, NICHD, NHLBI, and NIA of NIH.
In addition, we thank Eric Skaar (Department of Pathology, Microbiology, and Immunology, Vanderbilt University) and David Hachey (Mass Spectrometry Research Center, Vanderbilt University) for providing ICPMS services.
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02316-13.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott RD. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 3.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284:33949–33956. 10.1074/jbc.M109.070201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. 10.1016/j.chom.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10:525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis MS, Thomas CJ. 1997. Mutants in the CtpA copper transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microb. Pathog. 22:67–78. 10.1006/mpat.1996.0092 [DOI] [PubMed] [Google Scholar]
- 7.Schwan WR, Warrener P, Keunz E, Stover CK, Folger KR. 2005. Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int. J. Med. Microbiol. 295:237–242. 10.1016/j.ijmm.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 8.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. 2011. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 108:1621–1626. 10.1073/pnas.1009261108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. 2010. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 77:1096–1110. 10.1111/j.1365-2958.2010.07273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker J, Sitthisak S, Sengupta M, Johnson M, Jayaswal RK, Morrissey JA. 2010. Copper stress induces a global stress response in Staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl. Environ. Microbiol. 76:150–160. 10.1128/AEM.02268-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker J, Sengupta M, Jayaswal RK, Morrissey JA. 2011. The Staphylococcus aureus CsoR regulates both chromosomal and plasmid-encoded copper resistance mechanisms. Environ. Microbiol. 13:2495–2507. 10.1111/j.1462-2920.2011.02522.x [DOI] [PubMed] [Google Scholar]
- 12.Rowland JL, Niederweis M. 2012. Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis (Edinb.) 92:202–210. 10.1016/j.tube.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suwalsky M, Ungerer B, Quevedo L, Aguilar F, Sotomayor CP. 1998. Cu2+ ions interact with cell membranes. J. Inorg. Biochem. 70:233–238. 10.1016/S0162-0134(98)10021-1 [DOI] [PubMed] [Google Scholar]
- 14.Noyce JO, Michels H, Keevil CW. 2006. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 63:289–297. 10.1016/j.jhin.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 15.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. 2013. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect. Control Hosp. Epidemiol. 34:479–486. 10.1086/670207 [DOI] [PubMed] [Google Scholar]
- 16.Speer A, Shrestha TB, Bossmann SH, Basaraba RJ, Harber GJ, Michalek SM, Niederweis M, Kutsch O, Wolschendorf F. 2013. Copper-boosting compounds: a novel concept for antimycobacterial drug discovery. Antimicrob. Agents Chemother. 57:1089–1091. 10.1128/AAC.01781-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djoko KY, Paterson BM, Donnelly PS, McEwan AG. 2014. Antimicrobial effects of copper(II) bis(thiosemicarbazonato) complexes provide new insight into their biochemical mode of action. Metallomics 26:854–863. 10.1039/c3mt00348e [DOI] [PubMed] [Google Scholar]
- 18.Manning T, Mikula R, Lee H, Calvin A, Darrah J, Wylie G, Phillips D, Bythell BJ. 2014. The copper (II) ion as a carrier for the antibiotic capreomycin against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 24:976–982. 10.1016/j.bmcl.2013.12.053 [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Das O, Damle SG, Sharma AK. 2013. Isocitrate lyase: a potential target for anti-tubercular drugs. Recent Patents Inflamm. Allergy Drug Discov. 7:114–123. 10.2174/1872213X11307020003 [DOI] [PubMed] [Google Scholar]
- 20.Gingras BA, Suprunchuk T, Bayley CH. 1962. The preparation of some thiosemicarbazones and their copper complexes. Can. J. Chem. 40:1053–1959. 10.1139/v62-161 [DOI] [Google Scholar]
- 21.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. 1997. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 38:1155–1160 [PubMed] [Google Scholar]
- 22.Hasman H, Bjerrum MJ, Christiansen LE, Bruun Hansen HC, Aarestrup FM. 2009. The effect of pH and storage on copper speciation and bacterial growth in complex growth media. J. Microbiol. Methods 78:20–24. 10.1016/j.mimet.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 23.Nikaido H. 2009. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78:119–146. 10.1146/annurev.biochem.78.082907.145923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner D, Maser J, Lai B, Cai Z, Barry CE, III, Honer Zu Bentrup K, Russell DG, Bermudez LE. 2005. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J. Immunol. 174:1491–1500. 10.4049/jimmunol.174.3.1491 [DOI] [PubMed] [Google Scholar]
- 25.Underwood EJ. 1971. Trace elements in humans and animals, 4th ed. Academic Press, New York, NY [Google Scholar]
- 26.Munakata M, Kodama H, Fujisawa C, Hiroki T, Kimura K, Watanabe M, Nishikawa M, Tsuchiya S. 2012. Copper-trafficking efficacy of copper-pyruvaldehyde bis(N4-methylthiosemicarbazone) on the macular mouse, an animal model of Menkes disease. Pediatr. Res. 72:270–276. 10.1038/pr.2012.85 [DOI] [PubMed] [Google Scholar]
- 27.Iversen P, Beck B, Chen YF, Dere W, Devanarayan V, Eastwood BJ, Farmen MW, Iturria SJ, Iversen PW, Montrose C, Moore RA, Weidner JR. 2004. HTS assay validation. In Sittampalam GS, Gall-Edd N, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Lemmon V, Li Z, McGee J, McManus O, Minor L, Napper A, Riss T, Trask J, Jr, Weidner J. (ed), Assay guidance manual. Eli Lily & Co and National Center for Advancing Translational Science, Bethesda, MD [Google Scholar]
- 28.Chen SH, Lin JK, Liu SH, Liang YC, Lin-Shiau SY. 2008. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol. Sci. 102:138–149. 10.1093/toxsci/kfm292 [DOI] [PubMed] [Google Scholar]
- 29.Phillips M, Malloy G, Nedunchezian D, Lukrec A, Howard RG. 1991. Disulfiram inhibits the in vitro growth of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 35:785–787. 10.1128/AAC.35.4.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horita Y, Takii T, Yagi T, Ogawa K, Fujiwara N, Inagaki E, Kremer L, Sato Y, Kuroishi R, Lee Y, Makino T, Mizukami H, Hasegawa T, Yamamoto R, Onozaki K. 2012. Antitubercular activity of disulfiram, an antialcoholism drug, against multidrug- and extensively drug-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 56:4140–4145. 10.1128/AAC.06445-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson B. 1992. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. Suppl. 369:15–26 [DOI] [PubMed] [Google Scholar]
- 32.Grist R. 1992. External factors affecting imipenem performance in dried microdilution MIC plates. J. Clin. Microbiol. 30:535–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly JS, Dodge RA, Glew RH, Soja DT, DeLuca BA, Hebert S. 1997. Effect of zinc concentration in Mueller-Hinton agar on susceptibility of Pseudomonas aeruginosa to imipenem. J. Clin. Microbiol. 35:1027–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maresca A, Carta F, Vullo D, Supuran CT. 2013. Dithiocarbamates strongly inhibit the beta-class carbonic anhydrases from Mycobacterium tuberculosis. J. Enzyme Inhib. Med. Chem. 28:407–411. 10.3109/14756366.2011.641015 [DOI] [PubMed] [Google Scholar]
- 35.Velasco-Garcia R, Zaldivar-Machorro VJ, Mujica-Jimenez C, Gonzalez-Segura L, Munoz-Clares RA. 2006. Disulfiram irreversibly aggregates betaine aldehyde dehydrogenase—a potential target for antimicrobial agents against Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 341:408–415. 10.1016/j.bbrc.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 36.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426–2438. 10.1128/JB.187.7.2426-2438.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzegorzewicz AE, Kordulakova J, Jones V, Born SE, Belardinelli JM, Vaquie A, Gundi VA, Madacki J, Slama N, Laval F, Vaubourgeix J, Crew RM, Gicquel B, Daffe M, Morbidoni HR, Brennan PJ, Quemard A, McNeil MR, Jackson M. 2012. A common mechanism of inhibition of the Mycobacterium tuberculosis mycolic acid biosynthetic pathway by isoxyl and thiacetazone. J. Biol. Chem. 287:38434–38441. 10.1074/jbc.M112.400994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown JR, North EJ, Hurdle JG, Morisseau C, Scarborough JS, Sun D, Kordulakova J, Scherman MS, Jones V, Grzegorzewicz A, Crew RM, Jackson M, McNeil MR, Lee RE. 2011. The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg. Med. Chem. 19:5585–5595. 10.1016/j.bmc.2011.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menkissoglu O, Lindow SE. 1991. Relationship of free ionic copper and toxicity to bacteria in solutions of organic compounds. Phytopathology 81:1258–1263. 10.1094/Phyto-81-1258 [DOI] [Google Scholar]
- 40.Zevenhuizen LPTM, Dolfing J, Eshuis EJ, Scholten-Koerselman IJ. 1979. Inhibitory effects of copper on bacteria related to the free ion concentration. Microb. Ecol. 5:139–146. 10.1007/BF02010505 [DOI] [PubMed] [Google Scholar]
- 41.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. 2013. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 5:190ra181. 10.1126/scitranslmed.3006276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Z, Donnelly PS, Zimmermann M, Wedd AG. 2008. Transfer of copper between bis(thiosemicarbazone) ligands and intracellular copper-binding proteins. Insights into mechanisms of copper uptake and hypoxia selectivity. Inorg. Chem. 47:4338–4347. 10.1021/ic702440e [DOI] [PubMed] [Google Scholar]
- 43.Vavere AL, Lewis JS. 2007. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007:4893–4902. 10.1039/B705989B [DOI] [PubMed] [Google Scholar]
- 44.Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R, Marchio L. 2011. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J. Am. Chem. Soc. 133:6235–6242. 10.1021/ja109413c [DOI] [PubMed] [Google Scholar]
- 45.Lou JR, Zhang XX, Zheng J, Ding WQ. 2010. Transient metals enhance cytotoxicity of curcumin: potential involvement of the NF-kappaB and mTOR signaling pathways. Anticancer Res. 30:3249–3255 [PubMed] [Google Scholar]
- 46.John E, Fanwick PE, McKenzie AT, Stowell JG, Green MA. 1989. Structural characterization of a metal-based perfusion tracer: copper(II) pyruvaldehyde bis(N4-methylthiosemicarbazone). Int. J. Rad. Appl. Instrum. B 16:791–797. 10.1016/0883-2897(89)90163-3 [DOI] [PubMed] [Google Scholar]
- 47.Lahey ME, Gubler CJ, Cartwright GE, Wintrobe MM. 1953. Studies on copper metabolism. VII. Blood copper in pregnancy and various pathologic states. J. Clin. Invest. 32:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Festa RA, Thiele DJ. 2012. Copper at the front line of the host-pathogen battle. PLoS Pathog. 8:e1002887. 10.1371/journal.ppat.1002887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samanovic MI, Ding C, Thiele DJ, Darwin KH. 2012. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 11:106–115. 10.1016/j.chom.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crouch PJ, Hung LW, Adlard PA, Cortes M, Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, Laughton K, Volitakis I, Bush AI, Li QX, Masters CL, Cappai R, Cherny RA, Donnelly PS, White AR, Barnham KJ. 2009. Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 106:381–386. 10.1073/pnas.0809057106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cater MA, Pearson HB, Wolyniec K, Klaver P, Bilandzic M, Paterson BM, Bush AI, Humbert PO, La Fontaine S, Donnelly PS, Haupt Y. 2013. Increasing intracellular bioavailable copper selectively targets prostate cancer cells. ACS Chem. Biol. 8:1621–1631. 10.1021/cb400198p [DOI] [PubMed] [Google Scholar]
- 52.Aft RL, Lewis JS, Zhang F, Kim J, Welch MJ. 2003. Enhancing targeted radiotherapy by copper(II)diacetyl-bis(N4-methylthiosemicarbazone) using 2-deoxy-d-glucose. Cancer Res. 63:5496–5504 [PubMed] [Google Scholar]
- 53.Singh SK, Roberts SA, McDevitt SF, Weichsel A, Wildner GF, Grass GB, Rensing C, Montfort WR. 2011. Crystal structures of multicopper oxidase CueO bound to copper(I) and silver(I): functional role of a methionine-rich sequence. J. Biol. Chem. 286:37849–37857. 10.1074/jbc.M111.293589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grass G, Thakali K, Klebba PE, Thieme D, Muller A, Wildner GF, Rensing C. 2004. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J. Bacteriol. 186:5826–5833. 10.1128/JB.186.17.5826-5833.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopes SC, Ribeiro C, Gameiro P. 2013. A new approach to counteract bacteria resistance: a comparative study between moxifloxacin and a new moxifloxacin derivative in different model systems of bacterial membrane. Chem. Biol. Drug Des. 81:265–274. 10.1111/cbdd.12071 [DOI] [PubMed] [Google Scholar]
- 56.Iakovidis I, Delimaris I, Piperakis SM. 2011. Copper and its complexes in medicine: a biochemical approach. Mol. Biol. Int. 2011:594529. 10.4061/2011/594529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vardanyan Z, Trchounian A. 2010. The effects of copper (II) ions on Enterococcus hirae cell growth and the proton-translocating FoF1 ATPase activity. Cell Biochem. Biophys. 57:19–26. 10.1007/s12013-010-9078-z [DOI] [PubMed] [Google Scholar]
- 58.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349. 10.1073/pnas.0812808106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan SJ, Yan YK, Lee PP, Lim KH. 2010. Copper, gold and silver compounds as potential new anti-tumor metallodrugs. Future Med. Chem. 2:1591–1608. 10.4155/fmc.10.234 [DOI] [PubMed] [Google Scholar]
- 60.Komeda S, Casini A. 2012. Next-generation anticancer metallodrugs. Curr. Top. Med. Chem. 12:219–235. 10.2174/156802612799078964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.