Abstract
The antileishmanial activity of a series of bis-pyridinium derivatives that are analogues of pentamidine have been investigated, and all compounds assayed were found to display activity against promastigotes and intracellular amastigotes of Leishmania donovani and Leishmania major, with 50% effective concentrations (EC50s) lower than 1 μM in most cases. The majority of compounds showed similar behavior in both Leishmania species, being slightly more active against L. major amastigotes. However, compound VGP-106 {1,1′-(biphenyl-4,4′-diylmethylene)bis[4-(4-bromo-N-methylanilino)pyridinium] dibromide} exhibited significantly higher activity against L. donovani amastigotes (EC50, 0.86 ± 0.46 μM) with a lower toxicity in THP-1 cells (EC50, 206.54 ± 9.89 μM). As such, VGP-106 was chosen as a representative compound to further elucidate the mode of action of this family of inhibitors in promastigote forms of L. donovani. We have determined that uptake of VGP-106 in Leishmania is a temperature-independent process, suggesting that the compound crosses the parasite membrane by diffusion. Transmission electron microscopy analysis showed a severe mitochondrial swelling in parasites treated with compound VGP-106, which induces hyperpolarization of the mitochondrial membrane potential and a significant decrease of intracellular free ATP levels due to the inhibition of ATP synthesis. Additionally, we have confirmed that VGP-106 induces mitochondrial ROS production and an increase in intracellular Ca2+ levels. All these molecular events can activate the apoptotic process in Leishmania; however, propidium iodide assays gave no indication of DNA fragmentation. These results underline the potency of compound VGP-106, which may represent a new avenue for the development of novel antileishmanial compounds.
INTRODUCTION
Leishmaniasis, a broad-spectrum disease caused by protozoan parasites of the genus Leishmania, is one of the world's most neglected diseases, with 350 million people considered to be at risk of contracting leishmaniasis and more than 2 million new cases every year. Leishmaniasis has traditionally been classified into three main clinical forms, namely, visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL), which differ in terms of their immunopathologies and degrees of morbidity and mortality. Leishmania donovani causes the potentially fatal disease VL. In the absence of effective vaccines against leishmaniasis, the main means of controlling this disease is exclusively via chemotherapy. Current leishmaniasis treatments rely on a reduced arsenal of drugs, including pentavalent antimonials, amphotericin B, miltefosine, and paromomycin, all of which have drawbacks in terms of toxicity, efficacy, price, and inconvenient treatment schedules. To increase the therapeutic life span of these drugs and delay the emergence of resistance, the WHO has recommended combination therapy as a strategy to be implemented in clinical trials.
Pentamidine [1,5-bis(4-amidinophenoxy)pentane], which was first used for the treatment of sleeping sickness caused by Trypanosoma brucei, is currently a second-line drug for the treatment of visceral leishmaniasis. Pentamidine is actively transported into Leishmania promastigotes (1) and binds to nuclear and mitochondrial DNA (kinetoplasts), thereby hindering replication and transcription at the mitochondrial level (2). New diamidine and choline derivate dications have been developed recently in order to find new drugs with improved activity against leishmaniasis and lower toxicity (3–6).
We previously designed and synthesized a new set of bis-pyridinium compounds as inhibitors of the human choline kinase enzyme (7). This enzyme is a validated antitumor target, and all the above-mentioned compounds have shown a significant antiproliferative activity (7). Additionally, these compounds can be considered structural analogues of pentamidine in which the amidine moieties, which are protonated at physiological pH, have been replaced by positively charged nitrogen atoms in a pyridinium ring. In view of this structural resemblance and with the intention of identifying potential antileishmanial drugs, we analyzed the antileishmanial activities of a series of bis-pyridinium derivatives. Compound VGP-106 was identified as a representative compound that displayed a potent antileishmanial activity against L. donovani intracellular amastigotes. As the least cytotoxic of the set of compounds assayed for THP-1 cells, it was selected to further elucidate their mechanism of action in this protozoan parasite.
MATERIALS AND METHODS
Chemical compounds.
The synthesis of choline kinase inhibitors has been described previously (7). The compounds tested (see Table S1 in the supplemental material) were dissolved in dimethyl sulfoxide (DMSO) at 10 mM. Carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), amphotericin B, 4′,6-diamidino-2-phenylindole dilactate (DAPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phorbol 12-myristate 13-acetate (PMA), ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), propidium iodide (PI), and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO). Miltefosine was purchased from Æterna Zentaris (Frankfurt, Germany). Fluo4-AM, Pluronic F-127, Sytox green, bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)], Mitosox red, and JC-1 were purchased from Invitrogen (Carlsbad, CA). All the chemicals were of the highest quality available.
Leishmania cell lines and cultures.
Promastigotes of the reference strains Leishmania donovani (MHOM/IND/80/Dd8) and Leishmania major (MHOM/JL/80/Friedlin) for VL and CL, respectively, were grown at 28°C in RPMI 1640-modified medium (Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (iFBS) (Invitrogen) (8).
Drug susceptibility analysis in Leishmania promastigotes.
The susceptibility of Leishmania promastigote lines to the different bis-pyridinium compounds was determined after incubation at 28°C for 72 h in the presence of increasing concentrations of the compounds. The concentration of compound required to inhibit parasite growth by 50% (EC50) was calculated using the MTT colorimetric assay, as described previously (9). Miltefosine and amphotericin B were used as the standard antileishmanial agents.
Human myelomonocytic cell line (THP-1) culture and determination of cellular toxicity.
THP-1 cells were grown at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% iFBS, 2 mM glutamate, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells, at 3 × 104/well in 96-well plates, were differentiated to macrophages with 20 ng/ml of PMA treatment for 48 h followed by 24 h of culture in fresh medium (10). The cellular toxicity of all compounds was determined using the colorimetric MTT-based assay (9), as described previously for Leishmania promastigotes, except for the incubation temperature, which was 37°C in this case.
Susceptibility analysis in intracellular Leishmania amastigotes.
To determine the susceptibility of intracellular Leishmania amastigotes to these compounds, macrophage-differentiated THP-1 cells were infected at a macrophage/parasite ratio of 1:10. Infected-cell cultures were maintained at 37°C with 5% CO2 at different compound concentrations in RPMI 1640 medium plus 10% iFBS. After 72 h, macrophages were fixed for 20 min at 4°C with 2.5% paraformaldehyde in phosphate-buffered saline (PBS; 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 130 mM NaCl, and 2.6 mM KCl adjusted to pH 7) and permeabilized with 0.1% Triton X-100 in PBS for 30 min. Intracellular parasites were detected by nuclear staining with DAPI (Invitrogen). The percent infection and the mean number of amastigotes from infected macrophages were determined in 200 macrophages/well.
DNA constructs and transfection.
CEK (choline/ethanolamine kinase) and EK (ethanolamine kinase) from L. donovani (GeneDB L. donovani, accession codes LdBPK_351480.1 and LdBPK_271330.1, respectively) were isolated from the genomic DNA of L. donovani by PCR using sense and antisense primers 5′-atgatatcATGATGAGTGTGGACAATTC (lowercase indicates nucleotides added for gene cloning) and 5′-atggatccTCAGATAAGCTGCTTGTCTC for CEK and 5′-atcccgggATGGTGCAATTTACCGATATG and 5′-atggatccTCACAGGTTCTCTGAGCAC for EK. Restriction sites (underlined) were added for subsequent cloning. The resulting fragments were cloned into the Leishmania expression vector pXG-Hyg (11) and sequenced. L. donovani promastigotes were transfected with pXG-Hyg-CEK or pXG-Hyg-EK by electroporation in 2-mm-gap cuvettes at 450 V and 500 μF (BTX Electro Cell Manipulator 600). Transfectant parasites were selected for resistance to hygromycin B (500 μg/ml).
Gene expression.
Total RNA was extracted from different L. donovani lines using the High Pure RNA isolation kit (Roche Diagnostics GmbH, Germany) and transcribed into cDNA using the qScript cDNA synthesis kit (Quanta Biosciences, Inc.) following the manufacturer's instructions. The cDNA obtained was diluted (1:10 and 1:20) and amplified with the sense and antisense primers 5′-AGTTCCTGTCCAAGAAG and 5′-AGCTCAGGTGCGAGCAC for CEK, 5′-ACCTACAAGGACGAGTC and 5′-ATCTCGTACTCGCGAGA for EK, and 5′-GAAGTACACGGTGGAGGCTG and 5′-CGCTGATCACGACCTTCTTC for GADPH using 35 amplification cycles at an annealing temperature of 54°C. PCR products were electrophoresed on 1% agarose gel, stained with ethidium bromide and visualized under a UV illuminator, and the intensity relative to that of GADPH, used as an internal control, was measured.
Uptake of VGP-106.
Leishmania promastigotes (2 × 107 parasites/ml) were incubated in PBS with glucose (6 mM) and 100 μM VGP-106 at 28°C. Parasite aliquots were washed twice with PBS at different time points (5, 10, 30, and 60 min), and VGP-106 accumulation was determined fluorimetrically by recording an emission spectrum at 520 nm upon excitation at 340 nm using an Aminco-Bowman series 2 spectrofluorometer. Temperature dependence was determined at 28 and 4°C for 30 min.
Transmission electron microscopy (TEM) in L. donovani parasites.
Promastigotes were treated with 0.5 or 1 μM VGP-106 in culture medium for 24 and 48 h and then washed and fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2) overnight at 4°C. After fixing, the cell suspensions were washed in the same buffer and then postfixed in a mixture of 1% phosphate-buffered osmium tetroxide and 1% potassium ferrocyanide for 1 h prior to dehydration in a graded ethanol series and infiltration and embedding in EMbed 812 resin (EMS). Thin sections were cut and mounted on 300-mesh copper grids before staining with uranyl acetate and lead citrate (12). The samples were viewed using a Zeiss Libra 120 Plus TEM.
ATP measurements in L. donovani parasites.
ATP was measured using a CellTiter-Glo luminescence assay, which generates a luminescent signal proportional to the amount of ATP present, as described previously (13). Briefly, promastigotes (4 × 106/ml) were incubated at 28°C in culture medium for 24 and 48 h with 0.5 and 1 μM VGP-106. A 25-μl aliquot of each sample was then transferred to a 96-well plate, mixed with the same volume of CellTiter-Glo, and incubated in the dark for 10 min. The resulting bioluminescence was measured using an Infinite F200 microplate reader (Tecan Austria GmbH, Austria).
Analysis of mitochondrial membrane potential (ΔΨm) in L. donovani parasites.
Changes in ΔΨm were measured by flow cytometry using the JC-1 fluorescent marker, as described previously (14). Briefly, parasites (1 × 107 promastigotes/ml) were incubated with 0.5 or 1 μM VGP-106 for 24 and 48 h at 28°C in culture medium, then washed twice, resuspended in PBS, and further incubated at 28°C with 5 μM JC-1 for 10 min in HEPES-buffered saline (HBS) (21 mM HEPES, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, and 6 mM glucose, pH 7).
Plasma membrane permeabilization in L. donovani parasites.
Sytox green dye was used to assess plasma membrane integrity, as described previously (15). Briefly, parasites (1 × 107 promastigotes/ml) were treated with 10 or 30 μM VGP-106 for 1 and 3 h at 28°C in HBS, washed twice with HBS, and then incubated with 2 μM Sytox green (final concentration) for 15 min at 28°C. The parasites were subsequently transferred into a 96-well microplate (100 μl/well), and the fluorescence due to binding of the dye to intracellular nucleic acids was recorded using an Infinite F200 microplate reader. The control for maximum fluorescence was obtained by addition of 0.05% Triton X-100.
Determination of plasma membrane depolarization in L. donovani parasites.
The membrane potential-sensitive probe DiBAC4(3) was used to measure potential changes. Thus, parasites (1 × 107 promastigotes/ml) were incubated with and without 30 μM VGP-106 for 3 h in HBS at 28°C and then treated with 2 μM DiBAC4(3) for 10 min at 28°C, as described previously (15). Parasites treated with a 10 μM concentration of the depolarizing agent CCCP for 15 min were used as controls. DiBAC4(3) fluorescence was analyzed by flow cytometry.
Measurement of ROS production in L. donovani parasites.
The generation of mitochondrial ROS was measured using the cell-permeable fluorogenic probe Mitosox red, as described previously (16). Parasites (1 × 107/ml) were loaded with 5 μM Mitosox for 2 h at 28°C and then washed and resuspended in culture medium with 10 or 30 μM VGP-106 for 1 and 3 h at 28°C. After the washing, the fluorescence of oxidized Mitosox was measured by flow cytometry.
Analysis of free intracellular Ca2+ in L. donovani parasites.
Changes in cytosolic Ca2+ levels were monitored using the Ca2+-specific fluorescent probe Fluo4-AM, as described previously (15). Briefly, cells (1 × 107 promastigotes/ml) were incubated with 5 μM Fluo4-AM for 60 min at 28°C in HPMI medium (120 mM NaCl, 5 mM KCl, 400 μM MgCl2, 40 μM CaCl2, 10 mM HEPES, 10 mM NaHCO3, 10 mM glucose, and 5 mM Na2HPO4) supplemented with 0.02% pluronic acid F127. After incubation, the cells were washed and incubated with 10 or 30 μM VGP-106 in fresh HPMI medium, supplemented with 8 mM EGTA or unsupplemented. The fluorescence of Ca2+-bound Fluo4 was analyzed at 28°C using an Aminco-Bowman series 2 spectrofluorometer (excitation and emission wavelengths, 490 and 518 nm, respectively).
DNA content analysis in L. donovani parasites.
DNA content was analyzed by flow cytometry, as described previously (15). Briefly, parasites (1 × 107 promastigotes/ml) were incubated with or without 10 and 30 μM VGP-106 for 24 and 48 h at 28°C in culture medium, then washed twice with PBS, fixed in ice-cold methanol for 3 min on ice, resuspended in 500 μl of 1 μg/ml PI and 100 μg/ml RNase A in PBS, and incubated for 1 h in the dark at room temperature.
Statistical analysis.
Statistical significance was calculated using Student's t test. Differences were considered significant at a P value of <0.05.
RESULTS
Cytotoxicity of choline kinase inhibitors against Leishmania lines.
The antileishmanial activities of 10 choline kinase inhibitors (see Table S1 in the supplemental material) reported previously (7) were evaluated against promastigotes and intracellular amastigotes of L. donovani and L. major in order to identify potential candidates for further optimization as antileishmanial drugs. The results are shown in Table 1; miltefosine and amphotericin B were used as the reference antileishmanial drugs. Most assayed compounds exhibit a specific high activity against promastigotes and intracellular amastigotes of L. major, with EC50s for amastigotes of between 0.09 and 0.42 μM, except for compounds VGP-106 and VGP-118 (EC50, 13.07 and 6.21 μM, respectively). With regard to L. donovani, all assayed compounds displayed EC50s for promastigotes below 1 μM, except compound VGP-138 (EC50, 2.11 μM). Although these values were slightly higher for intracellular amastigotes, they were similar to those of the antileishmanial drug miltefosine.
TABLE 1.
| Compound | EC50 (μM) (SI)c |
THP-1 EC50 (μM) | |||
|---|---|---|---|---|---|
| Promastigotes |
Amastigotes |
||||
| L. major | L. donovani | L. major | L. donovani | ||
| VGP-106 | 21.55 ± 3.72 | 0.36 ± 0.09 | 13.07 ± 6.30 (15.8) | 0.86 ± 0.46 (240.2) | 206.54 ± 9.89 |
| VGP-114 | 0.47 ± 0.04 | 0.61 ± 0.09 | 0.10 ± 0.03 (1000.6) | 0.85 ± 0.04 (117.7) | 100.06 ± 8.57 |
| VGP-118 | 29.15 ± 5.73 | 0.65 ± 0.19 | 6.21 ± 1.02 (2.4) | 0.18 ± 0.03 (85.3) | 15.35 ± 3.99 |
| VGP-130 | 0.50 ± 0.07 | 0.73 ± 0.11 | 0.09 ± 0.02 (903.7) | 2.02 ± 0.05 (40.3) | 81.34 ± 10.65 |
| VGP-138 | 0.74 ± 0.19 | 2.11 ± 0.48 | 0.30 ± 0.16 (586.8) | 4.01 ± 0.43 (43.9) | 176.05 ± 20.75 |
| VGP-146 | 0.21 ± 0.06 | 0.33 ± 0.07 | 0.10 ± 0.04 (156.1) | 0.42 ± 0.01 (37.2) | 15.61 ± 3.26 |
| VGP-150 | 0.36 ± 0.11 | 0.77 ± 0.04 | 0.09 ± 0.03 (267) | 0.55 ± 0.16 (43.7) | 24.03 ± 5.42 |
| VGP-162 | 0.40 ± 0.08 | 0.35 ± 0.02 | 0.37 ± 0.03 (29.6) | 1.00 ± 0.08 (11.0) | 10.97 ± 2.41 |
| VGP-174 | 1.70 ± 0.01 | 0.34 ± 0.03 | 0.41 ± 0.05 (6.1) | 0.86 ± 0.03 (2.8) | 2.47 ± 0.05 |
| VGP-182 | 2.51 ± 0.01 | 0.92 ± 0.2 | 0.42 ± 0.12 (11.2) | 0.52 ± 0.12 (9.1) | 4.71 ± 0.23 |
| AmB | 0.32 ± 0.02 | 0.21 ± 0.01 | 0.24 ± 0.01 (59.7) | 0.28 ± 0.13 (51.1) | 14.32 ± 4.10 |
| Miltefosine | 16.65 ± 1.23 | 6.60 ± 1.57 | 10.61 ± 0.89 (2.5) | 0.88 ± 0.14 (30.5) | 26.86 ± 3.08 |
Parasites were grown as described in Materials and Methods for 72 h at 28°C (promastigotes) or 37°C (intracellular amastigotes) in the presence of increasing concentrations of compounds. Data are means ± standard deviations of three independent determinations.
THP-1 cells were grown as described in Materials and Methods for 72 h at 37°C, in the presence of increasing concentrations of compounds. Cell viability was determined using an MTT-based assay. Amphotericin B (AmB) and miltefosine were used as the standard antileishmanial agents. Data are means ± standard deviations from three independent experiments.
Selectivity indexes (SI) were calculated by dividing the EC50 for THP-1 by that for intracellular amastigotes.
Our analysis of the effect on THP-1 cells showed that bis-pyridinium derivatives (VGP-106, VGP-114, VGP-118, VGP-130, and VGP-138) are less cytotoxic than the bis-quinolinium counterparts (VGP-146, VGP-150, VGP-162, VGP-174, and VGP-182) (Table 1), with a higher selectivity index (ratio between EC50 THP-1 and EC50 in intracellular amastigotes) than miltefosine (Table 1).
VGP-106 (1,1′-(biphenyl-4,4′-diylmethylene)bis[4-(4-bromo-N-methylanilino)pyridinium] dibromide) (Fig. 1) was chosen as a representative compound to further investigate the mechanism of action of this new family of compounds. This compound showed strong activity against L. donovani and was the least cytotoxic of the set of compounds assayed for THP-1 cells.
FIG 1.
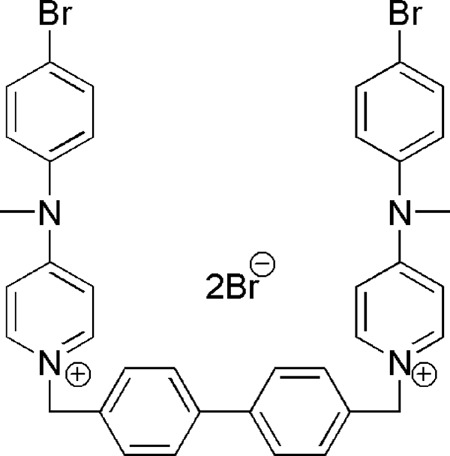
Structure of 1,1′-(biphenyl-4,4′-diylmethylene)bis[4-(4-bromo-N-methylanilino)pyridinium (VGP-106).
Drug susceptibility assay of L. donovani lines overexpressing CEK or EK.
Considering that all the above compounds were initially designed for inhibition of human choline kinase (ChoK) (7), we decided to study whether there is a correlation between their ChoK inhibitory activity and antileishmanial activities. The Leishmania genome includes two genes homologous to human ChoK, namely, the genes for choline/ethanolamine kinase (CEK) and ethanolamine kinase (EK). The corresponding proteins can be overexpressed in L. donovani promastigotes by transfecting the parasites with a plasmid carrying the Leishmania CEK or EK genes. Expression levels were tested by reverse transcription-PCR (RT-PCR) using GADPH as an expression control (Fig. 2). The susceptibility of transfected parasites to compound VGP-106 was determined in both promastigotes and intracellular amastigotes. As can be seen from Table 2, there are no significant differences between the EC50s for parasites overexpressing CEK or EK enzymes and those for control parasites. These results suggest that the mechanism of action of this compound in Leishmania is independent of the aforementioned enzymes. If this were not the case, overexpression of these enzymes would have resulted in an increased resistance to the inhibitory effect of the compound, and therefore an increase in the EC50.
FIG 2.
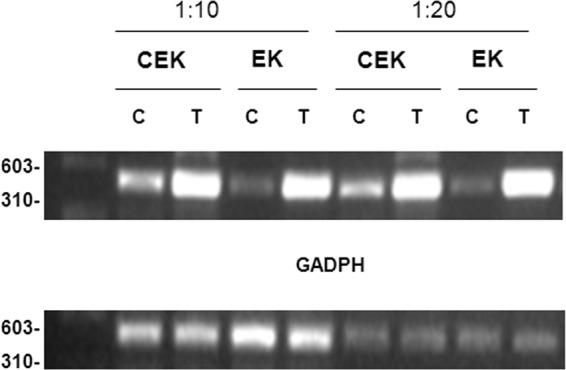
RT-PCR analysis of choline/ethanolamine kinase (CEK) and ethanolamine kinase (EK) gene expression levels in L. donovani. CEK and EK overexpression in L. donovani-transfected promastigotes (T) with respect to control promastigotes (C) was determined by RT-PCR at different cDNA dilutions (1:10 and 1:20). RT-PCR was carried out for 35 cycles and the products were run in 1% agarose gel as described in Materials and Methods. GADPH expression levels were used as loading controls. Data are representative of three independent experiments.
TABLE 2.
Susceptibility to VGP-106 of L. donovani lines overexpressing CEK or EKa
| Plasmid | EC50 (μM) |
|
|---|---|---|
| Promastigotes | Amastigotes | |
| pXG | 0.36 ± 0.09 | 0.45 ± 0.03 |
| pXG-CEK | 0.36 ± 0.09 | 0.42 ± 0.05 |
| pXG-EK | 0.43 ± 0.05 | 0.35 ± 0.03 |
Control (pXG) and transfected (pXG-CEK and pXG-EK) parasites were grown as described in Materials and Methods for 72 h at 28°C (promastigotes) or 37°C (intracellular amastigotes) in the presence of increasing concentrations of compound. Data are means ± SD of three independent determinations.
Uptake of VGP-106 in Leishmania lines.
To determine whether there were any differences in the uptake mechanism of VGP-106 between Leishmania lines that could explain differences in susceptibility to this compound, a spectrofluorometric assay taking advantage of the intrinsic fluorescence of this compound was carried out. A concentration of 100 μM VGP-106 allowed us to obtain significant fluorescence levels for experiments of drug uptake at different short time points.
As can be seen from Fig. 3A, VGP-106 uptake by the two Leishmania species reached saturation very quickly, and no significant differences in VGP-106 accumulation at 28°C and 4°C were observed (Fig. 3B), suggesting that uptake occurs by diffusion. Additionally, VGP-106 accumulation in L. donovani is only 18% higher than that in L. major, which does not explain the observed differences in susceptibility.
FIG 3.
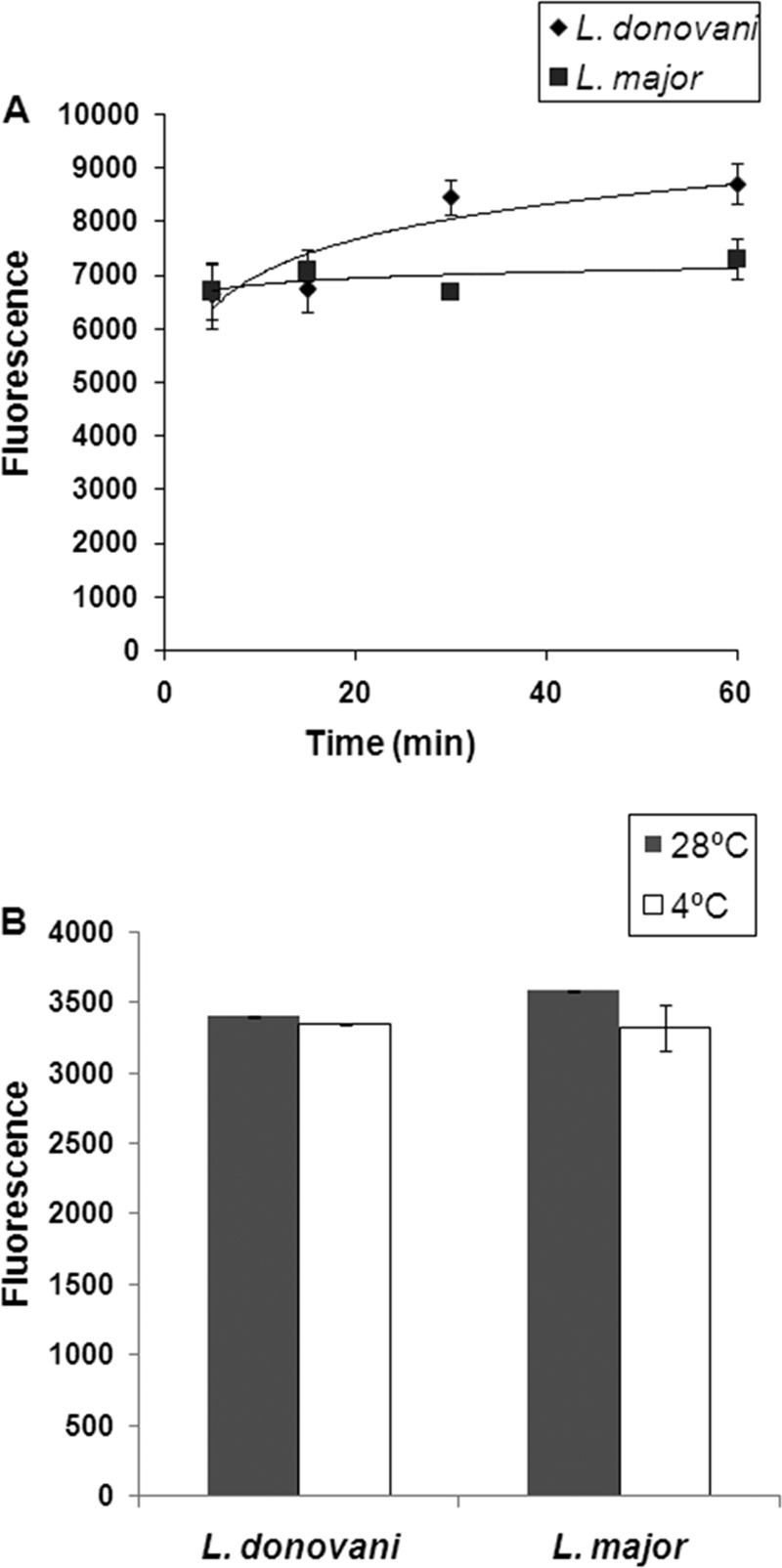
Uptake of VGP-106 in Leishmania lines. (A) Kinetics of VGP-106 uptake at 28°C in promastigotes of L. donovani and L. major lines. Samples were taken at different time points, and VGP-106 uptake was determined fluorimetrically as described in Materials and Methods. Data are representative of three independent experiments (means ± standard deviations [SD]). (B) Differences in VGP-106 accumulation in promastigotes of L. donovani and L. major at 28°C and 4°C. Data are representative of three independent experiments (means ± SD).
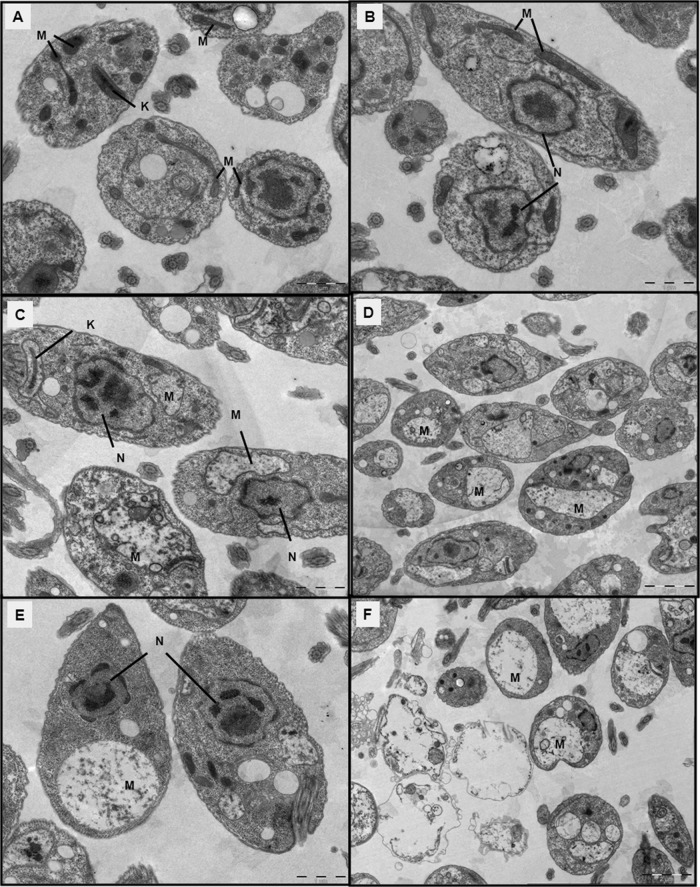
Effect of VGP-106 on the ultrastructural morphology of L. donovani.
The ultrastructural effect of VGP-106 in L. donovani promastigotes was studied by TEM. Parasites incubated with 0.5 μM for 24 or 48 h showed an intense swelling in the mitochondrion with loss of cristae and with no apparent kinetoplast alteration (Fig. 4C and E). This change was more evident at 1 μM, when the mitochondrion appeared as a huge vacuole covering a large fraction of the intracellular space. However, no further alterations were observed in other organelles or in the plasma membrane of the parasites. Furthermore, cell debris appeared after incubation with 1 μM at 48 h (Fig. 4F), suggesting a necrotic process.
FIG 4.
Ultrastructural effect of VGP-106 in L. donovani promastigotes by transmission electron microscopy (TEM). Ultrathin sections of control promastigotes of L. donovani (A and B) or promastigotes treated with 0.5 and 1 μM VGP-106 for 24 h (C and E) and 48 h (D and F) are shown. Mitochondria (M), kinetoplasts (K), and nuclei (N) are indicated. Scale bars: 1 μm (A, C, and E) or 2 μm (B, D, and F).
VGP-106 induces hyperpolarization of ΔΨm and decreases intracellular ATP levels in L. donovani.
To determine the effects of VGP-106 on mitochondrial function, we studied the variation in ΔΨm (Fig. 5). Parasites incubated with 0.5 and 1 μM VGP-106 for 24 and 48 h showed a significant increase in the JC-1 red/green fluorescence ratio compared with untreated parasites, indicating that this compound induces hyperpolarization of ΔΨm. The uncoupling reagent FCCP (10 μM, 10 min) was used as a control for fully depolarized promastigotes. As ΔΨm is essential for mitochondrial ATP synthesis, the intracellular ATP level was measured using a CellTiter-Glo luminescence assay, which generates a luminescent signal proportional to the amount of ATP. As observed, VGP-106 significantly reduces the intracellular ATP level in L. donovani promastigotes after treatment with either 0.5 or 1 μM VGP-106 for 24 h (Fig. 6).
FIG 5.
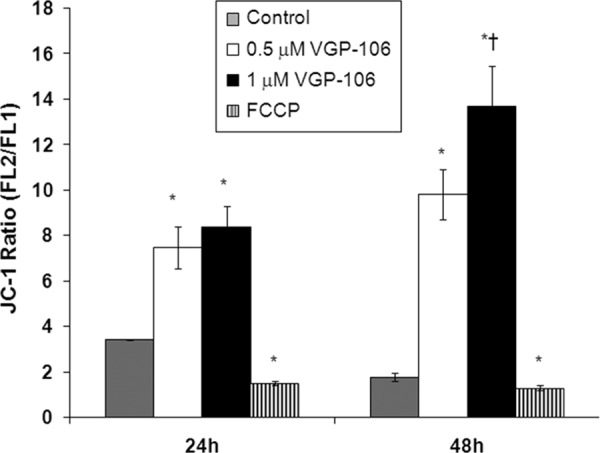
Effects of VGP-106 on the ΔΨm of L. donovani promastigotes. L. donovani promastigotes were treated with or without 0.5 and 1 μM VGP-106 for different time periods (24 and 48 h) and then incubated with 5 μM JC1 for 10 min for ΔΨm determination as described in Materials and Methods. The FL2/FL1 fluorescence ratio was measured by flow cytometry analysis. Parasites treated with 10 μM FCCP for 10 min were used as controls of full depolarization. Data are means ± SD of three independent experiments. Significant differences were determined using Student's t test (*, P < 0.01 versus parasite control; †, P < 0.01 versus 24 h of assay).
FIG 6.
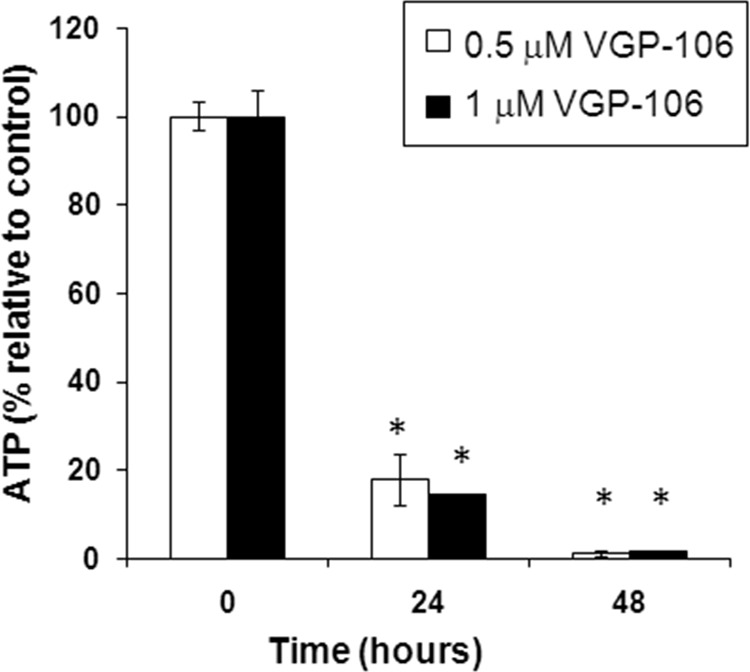
VGP-106 decreases intracellular ATP levels in L. donovani. Changes in intracellular ATP levels in L. donovani promastigotes treated with 0.5 or 1 μM VGP-106 for 24 and 48 h were determined using the CellTiter-Glo bioluminescence assay as described in Materials and Methods. Data are means ± SD of three independent experiments. Significant differences were determined using Student's t test (*, P < 0.01 versus untreated control cells).
VGP-106 does not affect the plasma membrane integrity of L. donovani.
To assess whether VGP-106 induces plasma membrane permeabilization, we first monitored the entry of the vital dye Sytox green into the cytoplasm of L. donovani promastigotes previously treated with VGP-106. The assays at the highest concentration (30 μM, 3 h) produce only 20% of the fluorescence increase obtained with 0.05% Triton X-100, which was used as a control that induces full permeabilization (Fig. 7A). Additionally, depolarization of the plasma membrane was measured using DiBAC4(3) as a fluorescence probe. After treatment with 30 μM VGP-106 for 3 h, no change was observed in the plasma membrane potential (Fig. 7B), confirming that this compound does not affect the plasma membrane integrity of parasites.
FIG 7.
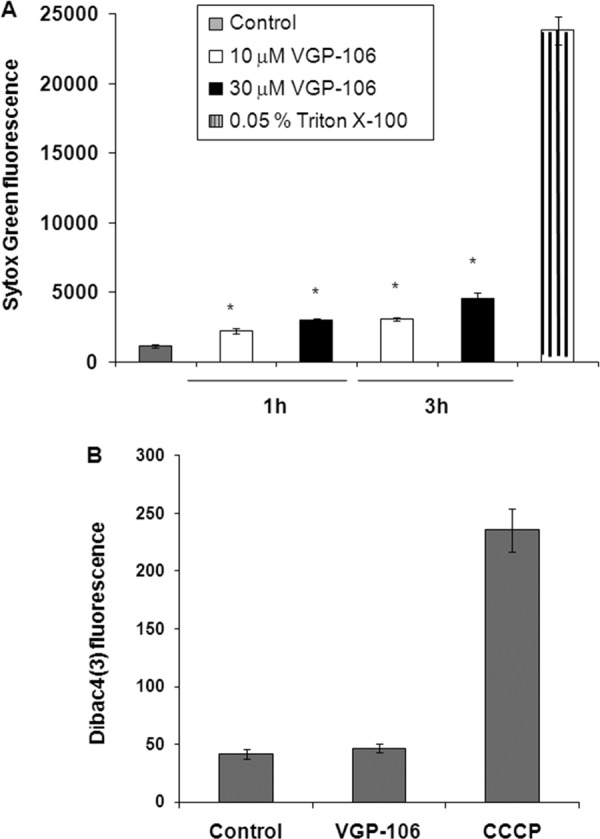
Effect of VGP-106 on the plasma membrane integrity of L. donovani. (A) The effect of VGP-106 on plasma permeability was determined by incubating promastigotes with or without 10 or 30 μM VGP-106 for 1 and 3 h at 28°C and then treating them with 2 μM Sytox green for 15 min at 28°C. Triton X-100 (0.05%) was used as a control for 100% permeabilization. Data are means ± SD from three independent experiments. Significant differences were determined using Student's t test (*, P < 0.01 versus controls). (B) The effect of VGP-106 on plasma membrane potential was determined by incubation with 30 μM compound for 3 h and then treatment with a 2 μM concentration of the specific plasma membrane potential probe DiBAC4(3) for 10 min at 28°C. Untreated parasites were used as the control, and treatment with 10 μM CCCP was used as 100% depolarization of the plasma membrane potential. Data are means ± SD from three independent experiments.
VGP-106 increases mitochondrial ROS production and cytosolic Ca2+ levels in L. donovani.
The modification of ΔΨm induced in Leishmania by a variety of drug treatments has been associated with the production of reactive oxygen species (ROS), which induce damage to the components of the electron transport chain, disrupt mitochondrial function, decrease cellular ATP levels, and produce cell death (17). The generation of mitochondrial ROS was measured using the cell probe Mitosox red, which selectively targets mitochondria and is oxidized by local superoxide. As can be seen from Fig. 8, VGP-106 produces a concentration- and time-dependent increase in ROS levels.
FIG 8.
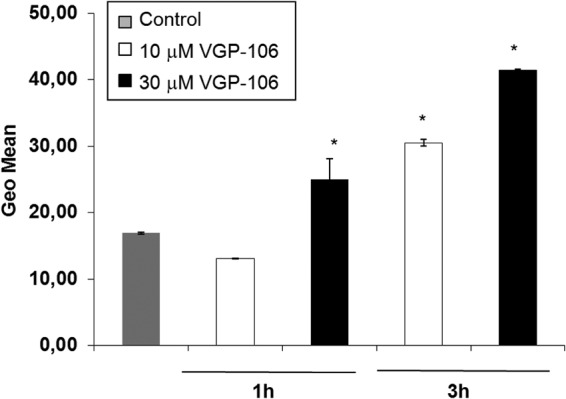
VGP-106 increases mitochondrial ROS production in L. donovani. Mitochondrial ROS levels were measured using the fluorescent dye Mitosox red after treating the promastigotes with or without 10 and 30 μM VGP-106 for 1 and 3 h at 28°C. Data are means ± SD of three independent experiments. Significant differences were determined using Student's t test (*, P < 0.01 versus control).
Mitochondrial damage is associated with both ROS production and variations in intracellular calcium homeostasis. Promastigotes treated with 10 and 30 μM VGP-106 showed higher cytosolic Ca2+ levels than untreated control parasites (Fig. 9A). To ascertain the source of the Ca2+ responsible for this effect, the experiment was repeated in the presence of EGTA to rule out the entry of external Ca2+ (Fig. 9B). Under these conditions, the fluorescence was significantly reduced, suggesting that VGP-106 induces an increase in the entry of external Ca2+.
FIG 9.
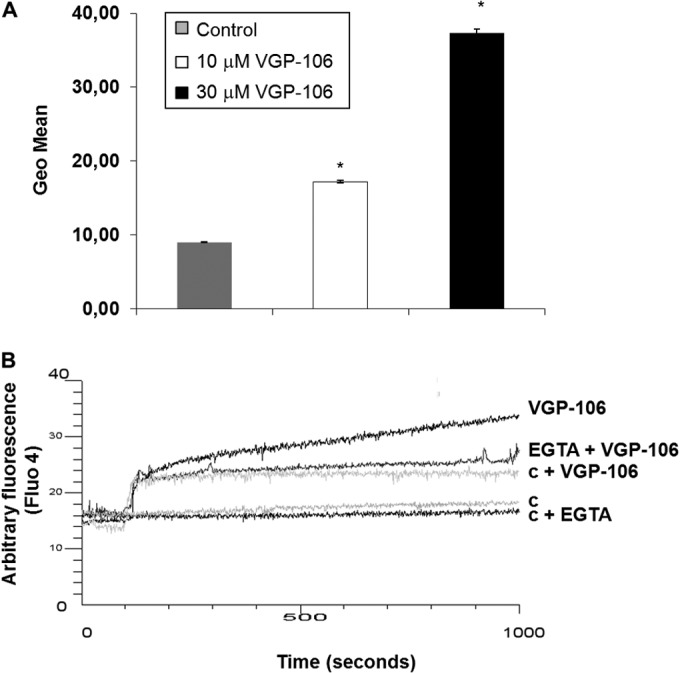
VGP-106 increases cytosolic Ca2+ levels in L. donovani. (A) Fluo-4-AM-preloaded promastigotes were treated with or without 10 or 30 μM VGP-106 for 30 min at 28°C, and the fluorescence of Fluo4-Ca2+ was determined. Data are means ± SD from three independent experiments. (B) Real-time Ca2+ determination assay carried out with promastigotes previously incubated with 5 μM Fluo4-AM treated with 30 μM VGP-106 and further analysis of the increased fluorescence. c, parasite control; c + VGP-106, fluorescence control of VGP-106 without parasites. The experiments were measured in the absence or presence of the Ca2+ chelator EGTA. Similar results were obtained in three independent experiments. The initial fluorescence increase is due to the intrinsic fluorescence of VGP-106 (trace c + VGP-106).
Effect of VGP-106 on the L. donovani cell cycle.
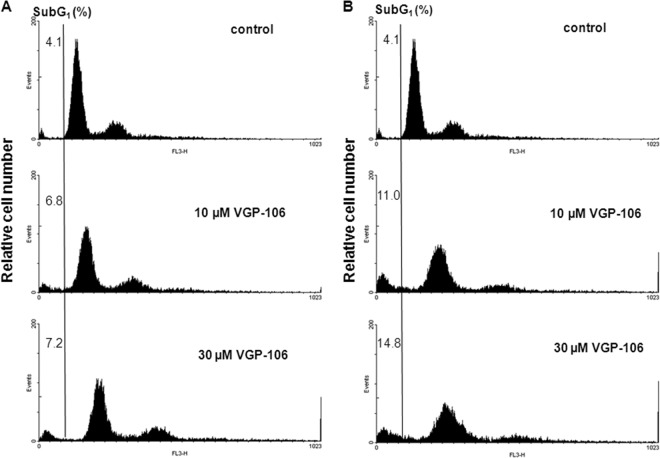
Since the elevation in cytosolic Ca2+, mitochondrial dysfunction, ROS generation, and reduction of intracellular ATP levels are all distinctive events during the progression of an apoptosis-like process, we examined whether VGP-106 produces DNA fragmentation, a key feature of apoptosis. We determined the hypodiploid DNA content in parasites by monitoring PI fluorescence using flow cytometry. After incubation of parasites with 10 and 30 μM VGP-106 during 24 and 48 h, there were no significant differences in the percentage of parasites with DNA in the sub-G1 phase with respect to untreated parasites (Fig. 10), indicating that there is no genomic DNA fragmentation. Therefore, our findings are consistent with nonprogrammed cell death.
FIG 10.
Effect of VGP-106 on the L. donovani cell cycle. DNA fragmentation was quantified by measuring the percentage of cells in the sub-G1 DNA region. The DNA content degradation profiles of promastigotes were determined by flow cytometry and PI staining. Parasites were incubated without (control) or with 10 and 30 μM VGP-106 for 24 h (A) and 48 h (B) and then loaded with PI, as described in Materials and Methods. The distribution of DNA content was analyzed by flow cytometry. Histograms are representative of three independent experiments, with 10,000 parasites analyzed per group.
DISCUSSION
Numerous dicationic compounds have been tested as antileishmanial drugs over the past few years (3–6). In this work, we determined the antileishmanial effect of a set of human choline kinase inhibitors with a bis-pyridinium-type structure (see Table S1 in the supplemental material) and provide a first insight into the antileishmanial mechanism of action of a promising lead compound.
All compounds assayed displayed antileishmanial activity against promastigotes and intracellular amastigotes of both L. donovani and L. major, with EC50s lower than 1 μM in most cases. Most compounds showed similar behavior in both Leishmania species, being slightly more active against L. major amastigotes. Nevertheless, compounds VGP-106 and VGP-118, which have a 4-(4-bromo-N-methylaniline)pyridinium group as the cationic head, exhibit significantly higher activity against L. donovani intracellular amastigotes.
VGP-106 was chosen as a representative compound in order to further elucidate the uptake and mode of action of this family of inhibitors. As a result, we have determined that the uptake of VGP-106 in Leishmania promastigotes quickly reached saturation and is a temperature-independent process, thereby suggesting that the compound crosses the parasite membrane by diffusion. An interesting observation was the differences in drug accumulation between L. donovani and L. major lines and the fact that these dissimilarities do not explain the different susceptibilities of Leishmania promastigotes and intracellular amastigotes to VGP-106; a similar situation has been described previously for sitamaquine (18). Considering that VGP-106 inhibits human ChoK (7), we decided to determine whether this set of bisquaternary derivatives produce parasite death by inhibiting homologous Leishmania enzymes. Several symmetrical biscationic compounds have previously demonstrated antimalarial activity resulting from inhibition of phosphatidylcholine biosynthesis (19). As described for eukaryotic cells, phosphatidylcholine is the main phospholipid of the plasma membrane in Leishmania, and there are two enzymes homologous to human ChoK in Leishmania: EK and CEK. In the VGP-106 susceptibility assay, results for Leishmania parasites overexpressing CEK and EK were similar to those for control parasites, meaning that these two enzymes are not implicated in the mode of action of this compound in Leishmania. This is in line with the fact that Leishmania is not auxotrophic for choline or ethanolamine (20), unlike Plasmodium, where ChoK inhibition leads to death of the parasite (21). Our results are consistent with those obtained by other authors for Trypanosoma brucei, indicating no changes in phospholipid metabolism after treatment with biscationic choline-derived analogues (6).
The TEM images showed an extremely swollen mitochondrion for parasites treated with compound VGP-106 compared to control parasites. In contrast, other organelles and the plasma membrane mostly appear intact, suggesting that this compound induces mitochondrial dysfunction in the parasites. The Leishmania mitochondrion is the target for a wide variety of leishmanicidal drugs, including some in clinical use, such as pentamidine (22) and miltefosine (23), and others at different stages of development, such as sitamaquine (15), tafenoquine (24), chalcones (25), and histatin 5 (26). Our study of the effect of VGP-106 on mitochondrial function under the same assay conditions showed a hyperpolarization of the mitochondrial membrane potential and a significant decrease of intracellular free ATP levels due to the inhibition of ATP synthesis. These findings suggest that VGP-106 may accumulate in the mitochondrion, thereby altering the mitochondrial function, as described previously for other positively charged compounds, such as dequalinium (27) and pentamidine (28), and other diamidine derivatives (29). In contrast to the observed hyperpolarization in ΔΨm induced by VGP-106, pentamidine, sitamaquine, and tafenoquine induce ΔΨm depolarization in Leishmania (15, 24, 30), whereas camptothecin induces an initial hyperpolarization followed by a depolarization of mitochondrial L. donovani (31). Leishmanial F0F1 ATPase plays a key role in increasing the mitochondrial membrane potential, which may depend on glycolytic ATP use by F0F1 ATPase in the reverse mode, as described for hyperpolarization in mammalian cells (31), for which it has been proved that hyperpolarization of the inner mitochondrial membrane leads to ROS production (32). Our experimental assays with the probe Mitosox red confirmed that VGP-106 induces mitochondrial ROS production in a time- and concentration-dependent manner. This ROS production triggers an increase in intracellular Ca2+ and subsequent steps in the apoptotic process in Leishmania. Interestingly, the Ca2+ pools involved may vary according to the stimulus. Thus, whereas oxidative stress induced by H2O2 involves mobilization from intra- and extracellular Ca2+ pools (33), only the intracellular pool is implicated after complex II poisoning with thenoyltrifluoroacetone plus pentamidine (34). An increase in cytosolic calcium homeostasis is known to be an essential initial event in cell death (35) and is related to ROS production and mitochondrial dysfunction. Although VGP-106 increases intracellular Ca2+ levels due to Ca2+ entry from the external medium, the question of whether the entry of external Ca2+ is due to unspecific and transitory membrane permeabilization by VGP-106 or to effects on Ca2+ channels remains unanswered. The entry of external Ca2+ in Leishmania apoptosis induced by external H2O2 (33), camptothecin (31), and curcumin (36) and mediated by specific channels activated by ROS or its derived metabolites inside the cells (37) was described previously.
All these molecular events activate programmed cell death with DNA nicking and fragmentation as the final outcome, both of which are characteristic of an apoptosis-like death in Leishmania. To determine whether VGP-106 produces apoptosis-like cell death, its effect on the cell cycle has been studied. However, the assays with PI indicate no signs of DNA fragmentation, contrary to the results obtained with pentamidine that binds to DNA (2). These results, together with the cell debris observed by TEM, confirm nonprogrammed cell death or necrosis. Recently, similar results were obtained with the iron chelator 2,2-dipyridyl, which leads to a multifactorial response in Leishmania braziliensis that results in cellular collapse, with a marked mitochondrial impairment and subsequent cell death not associated with DNA fragmentation (38).
In conclusion, our studies suggest that VGP-106 inhibits mitochondrial functionality, thereby inducing a rapid drop in intracellular ATP levels in Leishmania. At the same time, the increase in mitochondrial ROS production and elevation of intracellular Ca2+ leads to hyperpolarization of ΔΨm. Taken together, these biological events induced by VGP-106 trigger necrosis in Leishmania. The findings for VGP-106 reported herein support the view that this drug shows potency against Leishmania with sufficient activity for further in vivo studies. Preliminary in vivo experiments with VGP-106 using hamsters infected with L. infantum by oral or intraperitoneal administration showed no oral activity or high toxicity, respectively. Additional experiments are necessary for (i) evaluation of oral bioavailability, (ii) determination of the intrinsic toxicity, (iii) new experiments by intraperitoneal administration using lower dose of compound and increase number of days and intervals of treatment, and (iv) new routes of administration. Additionally, these studies may serve as a guide for the future design of VGP-106 analogues that are more specific and have higher antileishmanial activity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Spanish grants SAF2012-34267 (to F.G.) and SAF2011-28102 (to S.C.), by the Plan Andaluz de Investigación (Cod. BIO130), by FEDER funds from the EU to F.G. and S.C., and by an FPU fellowship (AP2009-3910) from the Ministerio de Educación (to V.G.P).
We thank Stephen M. Beverley (Washington University School of Medicine, St. Louis, MO, USA) for providing the vector pXG-Hyg, which was used throughout this research, and we thank Louis Maes (LMPH, University of Antwerp, Antwerp, Belgium) for the preliminary in vivo experiments with VGP-106.
Footnotes
Published ahead of print 5 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02481-13.
REFERENCES
- 1.Basselin M, Coombs GH, Barrett MP. 2000. Putrescine and spermidine transport in Leishmania. Mol. Biochem. Parasitol. 109:37–46. 10.1016/S0166-6851(00)00234-6 [DOI] [PubMed] [Google Scholar]
- 2.Sun T, Zhang Y. 2008. Pentamidine binds to tRNA through non-specific hydrophobic interactions and inhibits aminoacylation and translation. Nucleic Acids Res. 36:1654–1664. 10.1093/nar/gkm1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brendle JJ, Outlaw A, Kumar A, Boykin DW, Patrick DA, Tidwell RR, Werbovetz KA. 2002. Antileishmanial activities of several classes of aromatic dications. Antimicrob. Agents Chemother. 46:797–807. 10.1128/AAC.46.3.797-807.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosypal AC, Werbovetz KA, Salem M, Stephens CE, Kumar A, Boykin DW, Hall JE, Tidwell RR. 2008. Inhibition by dications of in vitro growth of Leishmania major and Leishmania tropica: causative agents of old world cutaneous leishmaniasis. J. Parasitol. 94:743–749. 10.1645/GE-1387.1 [DOI] [PubMed] [Google Scholar]
- 5.Bringmann G, Thomale K, Bischof S, Schneider C, Schultheis M, Schwarz T, Moll H, Schurigt U. 2013. A novel Leishmania major amastigote assay in 96-well format for rapid drug screening and its use for the discovery and evaluation of a new class of leishmanicidal quinolinium salts. Antimicrob. Agents Chemother. 57:3003–3011. 10.1128/AAC.02201-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim HM, Al-Salabi MI, El Sabbagh N, Quashie NB, Alkhaldi AA, Escale R, Smith TK, Vial HJ, de Koning HP. 2011. Symmetrical choline-derived dications display strong anti-kinetoplastid activity. J. Antimicrob. Chemother. 66:111–125. 10.1093/jac/dkq401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Pérez V, McSorley T, See Too WC, Konrad M, Campos JM. 2012. Novel 4-amino bis-pyridinium and bis-quinolinium derivatives as choline kinase inhibitors with antiproliferative activity against the human breast cancer SKBR-3 cell line. Chem. Med. Chem. 7:663–669. 10.1002/cmdc.201100505 [DOI] [PubMed] [Google Scholar]
- 8.Jackson PR, Lawrie JM, Stiteler JM, Hawkins DW, Wohlhieter JA, Rowton ED. 1986. Detection and characterization of Leishmania species and strains from mammals and vectors by hybridization and restriction endonuclease digestion of kinetoplast DNA. Vet. Parasitol. 20:195–215. 10.1016/0304-4017(86)90100-7 [DOI] [PubMed] [Google Scholar]
- 9.Kennedy ML, Cortés-Selva F, JM Pérez-Victoria Jiménez IA, González AG, Muñoz OM, Gamarro F, Castanys S, Ravelo AG. 2001. Chemosensitization of a multidrug-resistant Leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J. Med. Chem. 44:4668–4676. 10.1021/jm010970c [DOI] [PubMed] [Google Scholar]
- 10.El Fadili K, Imbeault M, Messier N, Roy G, Gourbal B, Bergeron M, Tremblay MJ, Légaré D, Ouellette M. 2008. Modulation of gene expression in human macrophages treated with the anti-leishmania pentavalent antimonial drug sodium stibogluconate. Antimicrob. Agents Chemother. 52:526–533. 10.1128/AAC.01183-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha DS, Schwarz JK, Turco SJ, Beverley SM. 1996. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77:57–64. 10.1016/0166-6851(96)02580-7 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208–212. 10.1083/jcb.17.1.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzano JI, Carvalho L, Perez-Victoria JM, Castanys S, Gamarro F. 2011. Increased glycolytic ATP synthesis is associated with tafenoquine resistance in Leishmania major. Antimicrob. Agents Chemother. 55:1045–1052. 10.1128/AAC.01545-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzano JI, García-Hernández R, Castanys S, Gamarro F. 2013. A new ABC half-transporter in Leishmania is involved in resistance to antimony. Antimicrob. Agents Chemother. 57:3719–3730. 10.1128/AAC.00211-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho L, Luque-Ortega JR, López-Martín C, Castanys S, Rivas L, Gamarro F. 2011. The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob. Agents Chemother. 55:4204–4210. 10.1128/AAC.00520-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira W, Leprohon P, Ouellette M. 2011. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2:e201. 10.1038/cddis.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alzate JF, Arias AA, Moreno-Mateos D, Alvarez-Barrientos A, Jimenez-Ruiz A. 2007. Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol. Biochem. Parasitol. 152:192–202. 10.1016/j.molbiopara.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 18.López-Martín C, Pérez-Victoria JM, Carvalho L, Castanys S, Gamarro F. 2008. Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 52:4030–4036. 10.1128/AAC.00964-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peyrottes S, Caldarelli S, Wein S, Perigaud C, Pellet A, Vial H. 2012. Choline analogues in malaria chemotherapy. Curr. Pharm. Des. 18:3454–3466. 10.2174/138161212801327338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zufferey R, Mamoun CB. 2002. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol. Biochem. Parasitol. 125:127–134. 10.1016/S0166-6851(02)00220-7 [DOI] [PubMed] [Google Scholar]
- 21.Ancelin ML, Vial HJ. 1986. Several lines of evidence demonstrating that Plasmodium falciparum, a parasitic organism, has distinct enzymes for the phosphorylation of choline and ethanolamine. FEBS Lett. 202:217–223. 10.1016/0014-5793(86)80690-1 [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee A, Padmanabhan PK, Sahani MH, Barrett MP, Madhubala R. 2006. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 145:1–10. 10.1016/j.molbiopara.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 23.Luque-Ortega JR, Rivas L. 2007. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 51:1327–1332. 10.1128/AAC.01415-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho L, Luque-Ortega JR, Manzano JI, Castanys S, Rivas L, Gamarro F. 2010. Tafenoquine, an anti-plasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrob. Agents Chemother. 54:5344–5351. 10.1128/AAC.00790-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Zhai L, Christensen SB, Theander TG, Kharazmi A. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023–2029. 10.1128/AAC.45.7.2023-2029.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luque-Ortega JR, van't Hof W, Veerman EC, Saugar JM, Rivas L. 2008. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 22:1817–1828. 10.1096/fj.07-096081 [DOI] [PubMed] [Google Scholar]
- 27.Weiss MJ, Wong JR, Ha CS, Bleday R, Salem RR, Steele GD, Chen LB. 1987. Dequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulation. Proc. Natl. Acad. Sci. U. S. A. 84:5444–5448. 10.1073/pnas.84.15.5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basselin M, Denise H, Coombs GH, Barrett MP. 2002. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob. Agents Chemother. 46:3731–3738. 10.1128/AAC.46.12.3731-3738.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanteri CA, Tidwell RR, Meshnick SR. 2008. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob. Agents Chemother. 52:875–882. 10.1128/AAC.00642-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vercesi AE, Docampo R. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284:463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK. 2004. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 11:924–936. 10.1038/sj.cdd.4401435 [DOI] [PubMed] [Google Scholar]
- 32.Brookes PS. 2005. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic. Biol. Med. 38:12–23. 10.1016/j.freeradbiomed.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee SB, Das M, Sudhandiran G, Shaha C. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717–24727. 10.1074/jbc.M201961200 [DOI] [PubMed] [Google Scholar]
- 34.Mehta A, Shaha C. 2004. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 279:11798–11813. 10.1074/jbc.M309341200 [DOI] [PubMed] [Google Scholar]
- 35.Golstein P, Kroemer G. 2007. Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 32:37–43. 10.1016/j.tibs.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 36.Das R, Roy A, Dutta N, Majumder HK. 2008. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13:867–882. 10.1007/s10495-008-0224-7 [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JM. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186:1–85. 10.1016/0076-6879(90)86093-B [DOI] [PubMed] [Google Scholar]
- 38.Mesquita-Rodrigues C, Menna-Barreto RF, Sabóia-Vahia L, Da-Silva SA, de Souza EM, Waghabi MC, Cuervo P, De Jesus JB. 2013. Cellular growth and mitochondrial ultrastructure of Leishmania (Viannia) braziliensis promastigotes are affected by the iron chelator 2,2-dipyridyl. PLoS Negl. Trop. Dis. 7:e2481. 10.1371/journal.pntd.0002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.