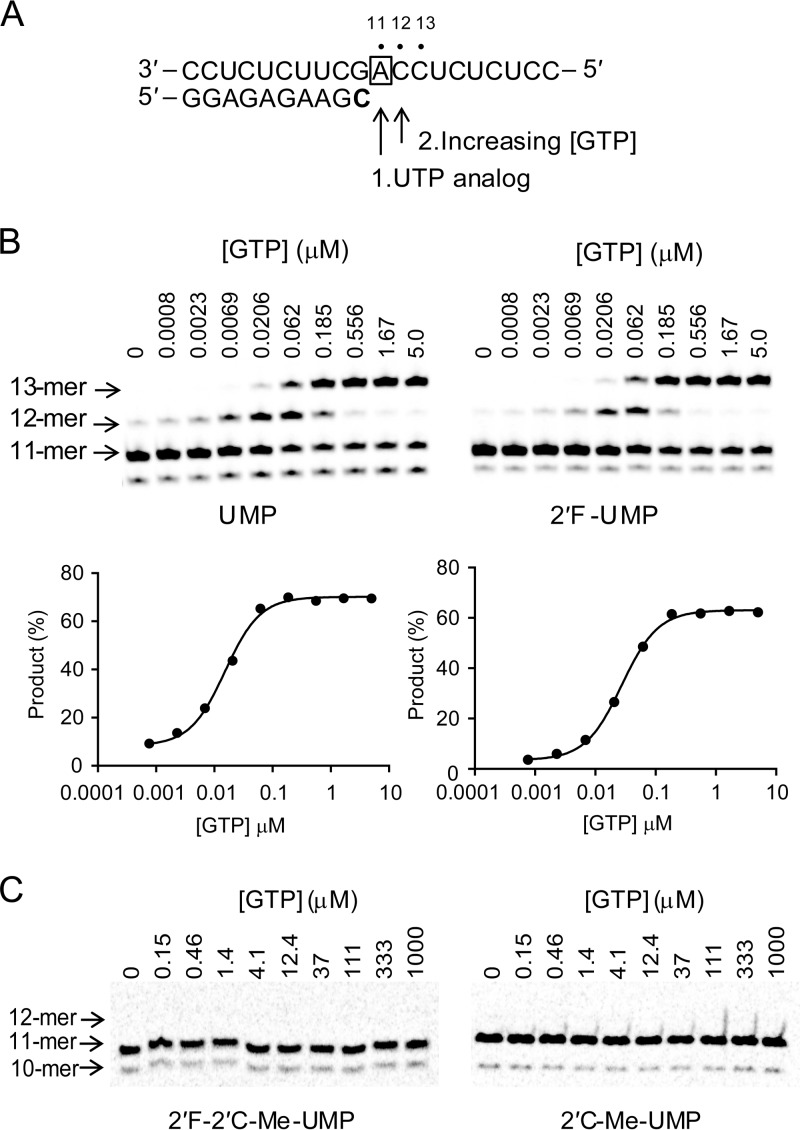
FIG 8.
Kinetics of next-correct-nucleotide incorporation. (A) Principle of the reaction. Once the stalled 10-mer EC was isolated, adding either the natural UTP (40 s) or a UTP analog (5 min) enabled the formation of the 11-mer product. After this step, GTP at various concentrations was added and reacted for a fixed time. The boldfaced “C” represents the radiolabeled nucleoside. The box around “A” represents the opposing nucleoside to the incoming nucleotide. (B) Gel image showing the GTP concentration-dependent incorporation of one or two consecutive GMPs into the 11-mer RNA containing a UMP or 2′F-UMP at its 3′ end. The sum of the 12- and 13-mer RNA products was expressed as a percentage of the initial amount of 11-mer. The percentage of GMP incorporation product was plotted as a function of GTP concentration after UMP (left) and 2′F-UMP (right). The data were fit to a sigmoid dose-response equation (equation 1) to obtain K1/2 values. The kpol/Kd values for GMP incorporation after UMP and 2′F-UMP were calculated from K1/2 values using equation 2, and the means ± standard deviations from more than two repeats are reported in Table 4. (C) GTP incorporation after 2′F-2′C-Me-UMP and 2′C-Me-UMP terminated RNA. Once the stalled 10-mer EC was isolated, it was mixed with 100 μM UTP analogs for 5 min to form the 11-mer product. After this step, GTP at various concentrations was added and reacted for 20 min. The gel image shows that no GMP incorporation (12-mer) was detected up to 1 mM GTP reaction for 20 min, resulting for both compounds in a discrimination level of >2 × 106 compared to GTP incorporation after natural UMP (Table 4).