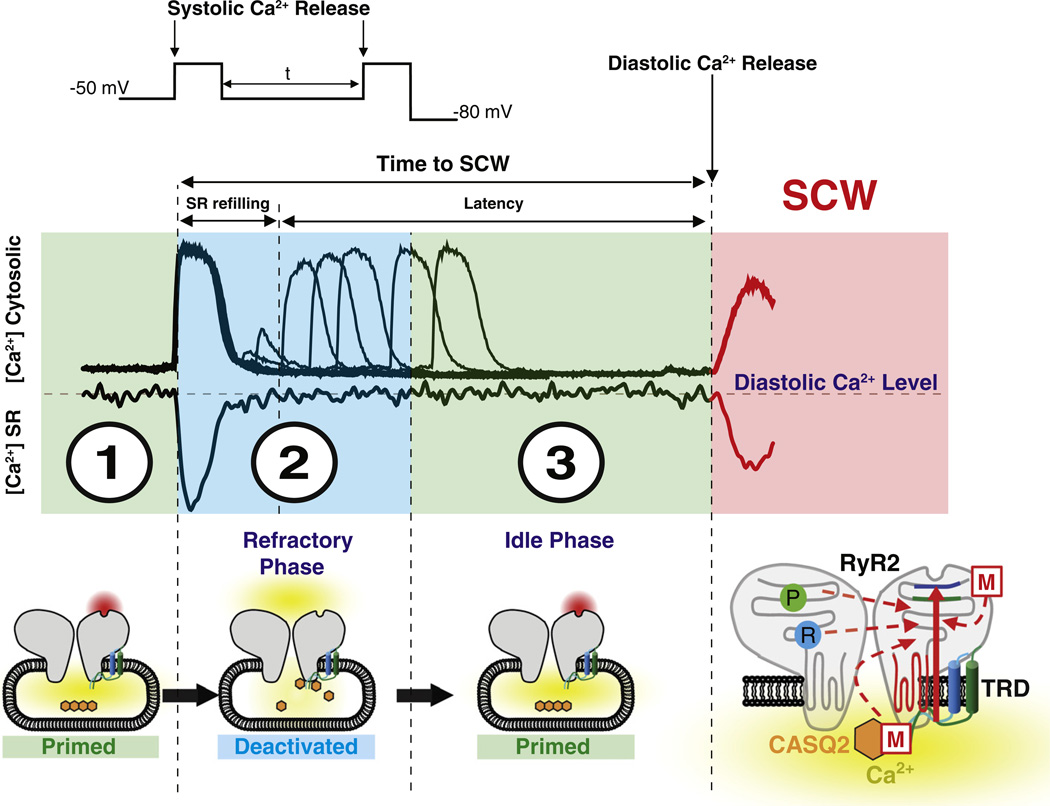
Fig. 1.
Prior to membrane depolarization, the SR Ca2+ interacts with CASQ2 functionally detaching it from the RyR2 complex rendering RyR2 primed for activation (primed cytosolic RyR2 activation site highlighted in red) by cytosolic Ca2+ (1). During RyR2 activation and the resulting systolic Ca2+ release, SR Ca2+ content is partially reduced thereby facilitating CASQ2 interaction with the RyR2 complex [via triadin (TRD) and/or junctin (JNT) (2). This interaction results in an allosteric inhibition of cytosolic activation site of the RyR2. The time between systolic SR Ca2+ release and spontaneous diastolic Ca2+ release is composed by refilling of SR Ca2+ store and latency during which the Ca2+ within the SR remains constant (2 and 3). The refractory phase reflects the time required for recovery of RyR2 from luminal Ca2+ deactivation and can be determined by a 2-pulse protocol (insets 1 and 2). At the completion of the refractory phase, refilling of the SR Ca2+ stores leads to dissociation of CASQ2 rendering RyR2 again functionally primed (primed cytosolic RyR2 activation site highlighted in red, 3) underlying thereby an idle phase during which stochastic activation of the recovered SR Ca2+ release sites triggers spontaneous diastolic Ca2+ waves (SCW). Phosphorylation (P) and redox modification (R) of RyR2 along with mutations in CASQ2 or the SR Ca2+ release complex (M) promote shortened recovery of RyR2 from luminal Ca2+ deactivation reducing thereby the refractory period and the time to spontaneous diastolic Ca2+ waves.