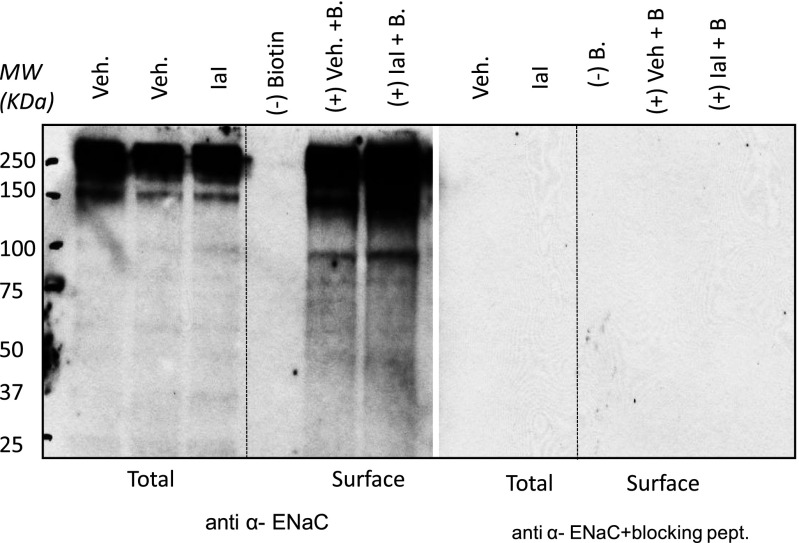
Figure 4.
IαI increase uncleaved ENaC. Western blots of total and surface proteins from rat ATII cells with an antibody against α-ENaC in the absence and presence of the immunizing peptide are shown. ATII cells were isolated from rat lungs, seeded on permeable supports, and cultured under liquid–liquid (3 d) and air–liquid (1–2 d) interfaces. IαI (0.1 mg/ml) was added on their apical surface. Four hours later, cells were lysed, and total and surface (biotinylated) proteins were isolated. Representative total (all proteins except those in the apical plasma membranes) and surface (biotinylated; proteins in apical surface) αENaC Western blots in the absence and presence of the immunizing peptide are shown. For surface labeling, 250 μg of protein were loaded with neutravidin beads as described in Materials and Methods. A total of 40 μg of protein were used. This experiment was repeated three times with similar results. Equal amounts of protein were loaded in each lane as verified by amido-black staining at the end of each experiment (data not shown). Notice the disappearance of all bands in the surface pool in the absence of biotin. The specificity of the anti-αENaC antibody was verified by the absence of staining in the presence of the immunizing peptide. IαI increased the intensity of the 95-kD band (uncleaved ENaC) in total and surface proteins. MW, molecular weight.