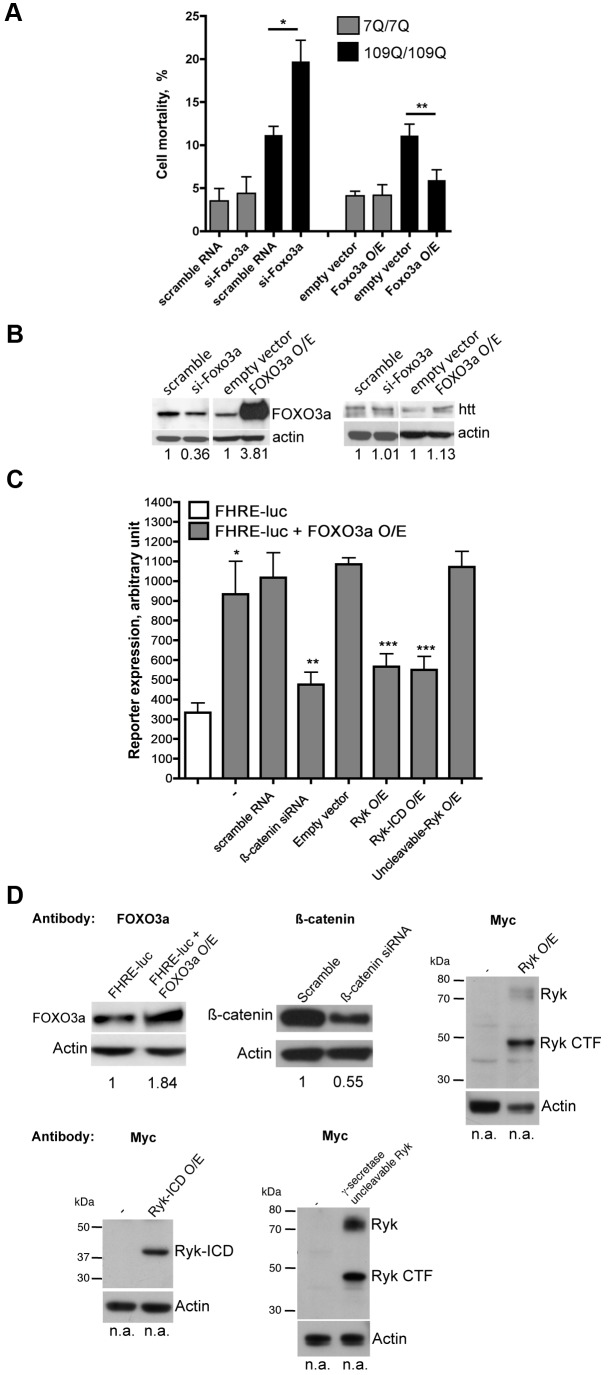
Figure 4. The Ryk ICD represses the transcriptional activity of FOXO3a, a protein that protects from mutant HTT.
(A) Foxo3a siRNA treatment enhances the mortality of mutant htt striatal cells subjected to serum deprivation, whereas FOXO3a overexpression (O/E) has the opposite effect. Data are mean ± SD (n = 4). *p<0.001 compared to scramble; **p<0.001 compared to empty vector control. (B) Representative Western blots showing decreased (si-Foxo3a) or increased (FOXO3a O/E) FOXO3a levels and no change in HTT protein levels. (C) FOXO transcriptional activity was measured in normal htt mouse striatal cells. Cells were cultured in normal conditions and co-transfected with a construct encoding FOXO3a together with the reporter FHRE-luciferase, which contains three canonical FOXO binding sites, and an internal Renilla luciferase reporter construct. Luciferase and Renilla luciferase activities were measured and the ratio (luciferase/Renilla luciferase)10,000 calculated. Data are mean ± SD of four independent experiments performed in triplicate. Treatment with β-catenin siRNA, full-length Ryk cDNA, and Ryk-ICD cDNA reduces luciferase activity to similar levels, whereas treatment with uncleavable Ryk showed no effect. *p<0.001 compared to FHRE-luc, **p<0.001 compared to scramble RNA and ***p<0.001 compared to FOXO3a O/E. Significance was tested using one-way ANOVA, with correction for multiple testing by Tukey's Multiple Comparison Test. (D) Representative Western blots showing increased levels of FOXO3a and decreased levels of β-catenin, and expression of Myc-tagged Ryk, Myc-tagged Ryk-ICD, and Myc-tagged γ-secretase–uncleavable Ryk (all proteins with a Myc tag at the C-terminal end). The Myc-tagged Ryk and γ-secretase–uncleavable Ryk proteins were detected as two fragments, one corresponding to the full-length Ryk precursor (Ryk) and one corresponding to a Ryk CTF (Ryk CTF) resulting from proteolytic cleavage in the extracellular domain near the transmembrane domain. The full-length Ryk precursor is less abundant for wild-type Ryk expression compared to mutant Ryk expression (see Results for the discussion of Ryk expression profiles).