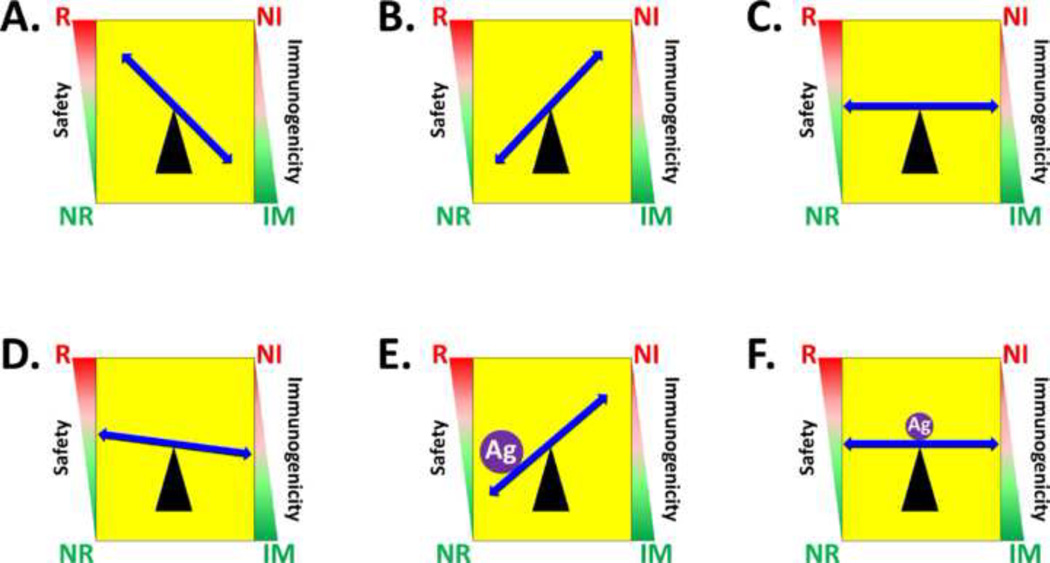
Figure 1. Balancing reactogenicity and immunogenicity in the development of live vaccines.
Both the safety and immunogenicity of a live bacterial strain are schematically represented in the left and right sides of each panel as a gradient of values. For safety, the gradient extends from a region of unacceptable reactogenicity (red zone denoted by “R”) to a region of acceptable non-reactogenicity (green zone denoted by “NR”); similarly, for immunogenicity, the gradient extends from a region of minimal immunogenicity (red zone designated as non-immunogenic, “NI”) to the desired region of immunogenicity (green zone designated as immunogenic, “IM”). Panels A–C schematically represent construction of a properly attenuated vaccine strain; and Panels D–F represent construction of a carrier vaccine expressing additional protective antigens from unrelated pathogens. In this graphic, pathogenic organisms are represented by Panel A, over-attenuated vaccine strains engineered from pathogens are represented by Panel B, and properly engineered attenuated vaccines are represented by Panel C. Given that expression of a foreign antigen can elevate metabolic burden and increase attenuation, starting with a slightly reactogenic vaccine candidate (Panel D) and over-expressing a foreign antigen (purple filled circle labeled “Ag”) will tip the balance and result in a safe but non-immunogenic live vaccine (Panel E), while expression of sufficient levels of foreign antigen to elicit immunogenicity without over-attenuating the carrier strain will yield both a safe and immunogenic carrier vaccine (Panel F).