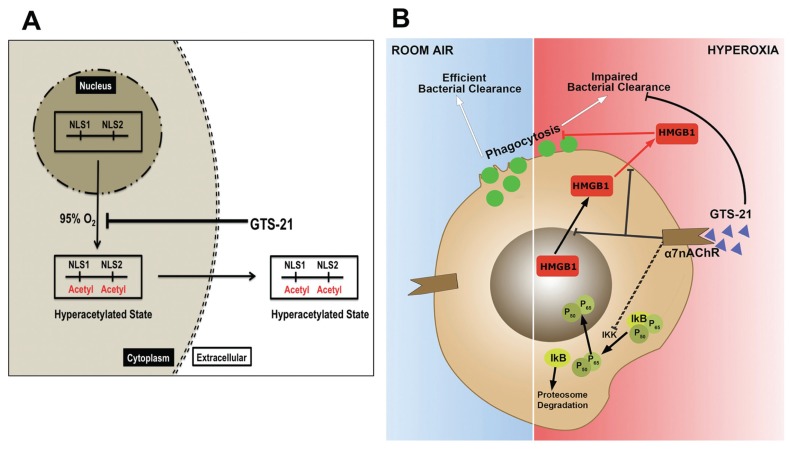
Figure 6.
Hypothesized pathway of GTS-21–inhibited hyperoxia-induced HMGB1 release (A) and improved hyperoxia-compromised macrophage function in bacterial clearance (B). Hyperoxia induces the hyperacetylation of NLS1 and NLS2 sites on HMGB1, thus causing its cytoplasmic translocation and subsequent release from cells. GTS-21 inhibits hyperoxia-induced HMGB1 cytoplasmic translocation by inhibiting the hyperacetylation of HMGB1 (A). Under room air conditions, alveolar macrophages maintain normal phagocytic activity and efficiently clear bacteria. In macrophages exposed to hyperoxia, NF-κB is translocated into the nucleus, whereas HMGB1 translocates from the nucleus into the cytoplasm and subsequently into the extracellular milieu, leading to the impairment of phagocytosis and bacterial clearance by macrophages. GTS-21 can efficiently attenuate hyperoxia-impaired bacterial clearance by suppressing hyper-acetylation of HMGB1, which leads to its translocation into the cytoplasm and subsequent accumulation of extracellular HMGB1. GTS-21 also inhibits NF-κB translocation into the nucleus, which may prevent hyperacetylation of HMGB1 and subsequent translocation and release into the extracellular milieu.