Abstract
Increased sialylation and β1,6-branched oligosaccharides has been associated with a variety of structural changes in cell surface carbohydrates, most notably in tumorigenesis. Lectins are defined as proteins that preferentially recognize and bind carbohydrate complexes protruding from glycolipids and glycoproteins. This interaction with carbohydrates can be as specific as the interaction between antigen and antibody. Due to this type of interaction lectins have been used as experimental auxiliary tools in histopathological diagnosis of cancer. This study was designed to evaluate the differential expression of sialic acids and β1,6-N-acetylglucosaminyltransferase V (MGAT5) in invasive (IDC) and in situ (DCIS) ductal carcinoma of the breast and its possible application as prognostic biomarkers. A possible transition between pre-malign and malign lesions was evaluated using DCIS samples. Biopsies were analyzed regarding the expression of MUC1, p53, Ki-67, estrogen receptor, progesterone receptor, HER-2 and MGAT5. α2,6-linked sialic acids residues recognized by SNA lectin was overexpressed in 33.3% of IDC samples and it was related with Ki-67 (p=0.042), PR (p=0.029), lymphnodes status (p=0.017) and death (p=0.011). Regarding survival analysis SNA was the only lectin able to correlate with specific-disease survival and disease-free survival (p=0.024 and p=0.041, respectively), besides, it presents itself as an independent variable by Cox Regression analysis (p= 0.004). Comparing IDC and DCIS cases, only SNA showed different staining pattern (p=0.034). The presence of sialic acids on tumor cell surface can be an indicative of poor prognosis and our study provides further evidence that SNA lectin can be used as a prognostic probe in IDC and DCIS patients.
Keywords: Sialic acids, lectins histochemistry, MAL-II, SNA, prognosis
Introduction
Breast cancer is the most common cancer among women worldwide [1]. In Brazil the estimative rate for 2014 is 57.120 of new breast cancer cases with more than 12.000 deaths [2] while in the USA about 300.000 cases of breast cancer is expect to be newly diagnosed in the same year [1]. This high mortality rate is probably because the disease is still diagnosed in advanced stages. Even with great advances in diagnosing and treatment methods, both clinical and scientific problems remain unsolved [3].
Aberrant and deregulated protein glycosylation is a well-established event in the process of oncogenesis and cancer progression [4,5]. Such post-translational changes can affect many cellular processes such as cell-cell and cell-matrix recognition, adhesion, motility, control of membrane permeability and molecular recognition [6,7].
The glycocode in glycoconjugates (glycoproteins or glycolipids) is orchestrated by glycosyltransferases and glycosidases, responsible for the synthesis and the breakdown of glycoconjugates, respectively, being their aberrant expression often related to the regulation of biological tumors behaviors [6-8]. These enzymes show to be useful cancer biomarkers for predicting clinical outcome in cancer [9].
One of the most common carbohydrates that participate of carcinogenesis, invasion and metastasis is the sialic acid [8]. Tumor cells relationships with themselves and with surrounding tissue are related to aberrant expression of terminal sialic acids; in particular α2,3 and α2,6-linked sialic acids [8,10]. Tumorigenesis-involved proteins can present overexpression of sialic acids residues as Integrins α5, αv and β1 [11,12], epidermal growth factor receptor (EGFR), Lamp-1, MUC1 and N-Cadherin [13] and they may contribute directly with altered growth control, adhesion, epithelial-mesenchymal transition and metastasis [14].
β1,6-N-acetylglucosaminyltransferase V (MGAT5; or GnT-V commonly used to refer the gene) catalyzes β1,6 branching of N-acetylglucosamine residues (GlcNAc) on asparagine N-linked oligosaccharides (N-glycan) of cell proteins [15]. MGAT5 and β1,6-GlcNAc branched N-glycans are tumor-associated glycoproteins commonly increased in malign ones, and their higher levels were already correlated with disease progression in colorectal cancer [16] and lymph node metastasis in breast cancer [17]. N-acetylglucosamine residues can be recognized by a variety of lectins and according to Cummings et al. [18], Phaseolus vulgaris leucoagglutinating (PHA-L) binds specifically to mature MGAT5 products (β1,6-GlcNAc branched N-glycans) and, therefore, it has been used as a probe for MGAT5-modified glycans.
Kuzmanov, Kosanam and Diamandis [4] presented a series of technical and biological obstacles to reflect the status of a glycoprotein biomarker and there is only a very limited set of tools capable of performing this task, and lectins are one of these useful tools. These oligomeric proteins have saccharide-binding sites that can recognize and bind specific saccharides in glycoconjugate as those in cancer cells which often display altered surface glycoproteins [19]. Lectin histochemistry has been used to investigate the cell surface carbohydrates in normal and transformed tissues [20-22]. Its application was documented, for example, by Dosaka-Akita et al. [23] who used PHA-L to evaluate β1,6-branches on N-glycans of glycoproteins in non-small cell lung carcinoma; López-Morales et al. [24], applied Maackia amurensis agglutinin (MAA), which interacts with α2,3-linked sialic acid, and Sambucus nigra agglutinin (SNA), specific for α2,6-linked sialic acid, to examine the expression and distribution of sialic acid in different stages of cervical neoplasia.
Increased sialylation and β1,6-branched oligosaccharides has been associated with a variety of structural changes in cell surface carbohydrates, including tumorigenesis. This study was designed to evaluate the differential expression of sialic acids and β1,6-N-acetylglucosaminyltransferase V in invasive (IDC) and in situ (DCIS) ductal carcinoma of the breast and its possible application as prognostic biomarkers. A possible transition between pre-malign and malign lesions was evaluated using DCIS samples.
Materials and methods
Patients’ files
Formalin-fixed paraffin embedded samples of invasive ductal carcinomas (IDC, n=225 - sizes ranging between 5-180 mm (mean 35 mm) were randomly chosen from the Biopsies Bank of the Department of Pathology of Ribeirão Preto Medical School at University of São Paulo, Brazil. The average age of patients included in this study was 55 years old (range 25-85 years). Lectin histochemistry with SNA and MAL-II (specific to α2,6- and α2,3-linked sialic acid residues) and PHA-L (specific to β1,6-N-acetylglucosamine) and immunohistochemistry (ER, PR, Ki67, P53, HER-2, MUC1 and β1,6-N-acetylglucosaminyltransferase V) were performed. In ductal carcinoma in situ samples (DCIS, n=67 - age ranging from 24 to 83 year-old, mean=51 year-old) SNA, MAL-II and PHA-L stainings as well as MGAT5, ER, PR, HER-2 were evaluated for comparing with IDC samples. Clinic histopathology data (age, menopausal status, tumor size, regional lymph nodes metastasis, recurrence, distant metastasis and death) were used for patient’s characterization. Patients were selected based on their histopathologic diagnosis and reviewed by a breast Pathologist (ARS) to confirm the diagnosis. As exclusion criteria the patients who received any oncology treatment before the diagnostic biopsy procedure were excluded of the study. The protocol used in this study was in accordance with the ethical guidelines of the Declaration of Helsinki and it was previously approved by the Ethic Committee.
Tissue microarray - TMA
Core biopsies (diameter, 1 mm) were punched from representative regions of each formalin-fixed and paraffin-embedded samples (IDC=225 and DCIS=67) and were arrayed into a new paraffin block using a Manual Tissue Arrayer I (Beecher Instruments, Silver Spring, USA). Tissue sections (3 μm-thick) were cut from the TMA paraffin block using a paraffin tape-transfer system (Instrumedics, Saint Louis, USA) and placed in glass slides.
Immunohistochemistry
It was performed using the Biocare Medical Mach 4 Universal Polymer Detection (Concord, California, USA) according to Ribeiro-Silva et al. [25] and dos-Santos et al. [26]. The dilution and clone specification of the primary antibodies used in this study are presented in Table 1. Antibody recognition reaction was visualized using 3,3’-diaminobenzidine (DAB) followed by hematoxylin counterstaining. Normal liver samples were used as positive control for MGAT5. IDC cases previously known to be positive for Ki67, ER, PR, p53, HER-2 were used as positive controls for each reaction. Negative controls were prepared omitting the primary antibody.
Table 1.
Immune and lectin histochemistry reagents
| Antibody*/Lectin** | Dilution/Concentration | Clone/Source | Manufacturer |
|---|---|---|---|
| MGAT5 | 1:50 | Polyclonal | Sigma-Aldrich, USA |
| MUC1 | 1:50 | 695 | Biocare Medical Concord, CA, USA |
| Ki67 | 1:100 | MMI | Novocastra, Newcastle upon Tyne, UK |
| ER | 1:100 | 6F11 | Novocastra, Newcastle upon Tyne, UK |
| PR | 1:100 | 16 | Novocastra, Newcastle upon Tyne, UK |
| p53 | 1:50 | DO-7 | Novocastra, Newcastle upon Tyne, UK |
| HER-2 | 1:100 | CB11 | Novocastra, Newcastle upon Tyne, UK |
| MAL-II | 20 μg/mL | Maackia amurensis | Vector Labs, Burlingame, CA, USA |
| SNA | 20 μg/mL | Sambucus nigra | Vector Labs, Burlingame, CA, USA |
| PHA-L | 20 μg/mL | Phaseolus vulgaris | Vector Labs, Burlingame, CA, USA |
ER: estrogen receptor; PR: progesterone receptor; Human Epidermal growth factor Receptor 2.
MAL-II: Maackia amurensis agglutinin; SNA: Sambucus nigra agglutinin; PHA-L: Phytohemaglutinin-L.
Lectin histochemistry
Sambucus nigra agglutinin (SNA), Maackia amurensis agglutinin (MAL-II) and Phytohemaglutinin-L (PHA-L) conjugated to biotin were used in this study. These lectins recognize α2,6-linked sialic acid, α2,3-linked sialic acid and β1,6-GlcNAc branched N-glycans, respectively. Tissue sections (3 μm-thick) were deparaffinized in xylene, rehydrated in ethanol, incubated with a 0.3% hydrogen peroxide-methanol solution for 30 minutes at 25°C and treated with a 0.1% trypsin solution for 15 min at 37°C. For PHA-L, after trypsin treatment, sections were also treated with a neuroaminidase solution (0.1 U/mL) from Clostridium perfringens (Sigma Aldrich, Missouri, USA) for 1 hour at 37°C. After trypsin and/or neuraminidase treatment slices were incubated with 2% BSA (bovine serum albumin) solution and then incubated, separately, with the lectins solutions (20 μg/mL) overnight at 4°C. Slices were then incubated with streptavidin-peroxidase polymer (Sigma Aldrich, Missouri, USA) for 45 min at 25°C. A 100 mM phosphate buffer solution (PBS), supplemented with 150 mM NaCl, pH 7.4 was used for washes between each protocol steps and solution preparation when not indicated elsewhere. Lectin staining was revealed with 3,3’-diaminobenzedine (DAB) followed by hematoxylin counterstaining. Lectins staining negative controls were carried out replacing lectin by PBS. Table 1 presents the concentration and source specifications of the lectins used in this study.
Immuno- and lectin histochemistry evaluation
Immune stained slides were scored as positive as following: Ki67 (>14% of cancer cells [27]), ER and PR (>1% of cancer cells [28]), p53 (>5% of cancer cells [29]), MUC1 (>10% of cancer cells [30]). HER-2 was evaluated according to the ASCO/CAP HER-2 Guideline update [31] as follow: IHC 3+ defined based on circumferential membrane staining that is complete, intense; IHC 2+ defined based on circumferential membrane staining that is incomplete and/or weak/moderate and within >10% of the invasive tumor cells; or complete and circumferential membrane staining that is intense and within ≤10% of the invasive tumor cells; IHC 1+ defined by incomplete membrane staining that is faint/barely perceptible and within >10% of the invasive tumor cells; IHC 0 defined by no staining observed or membrane staining that is incomplete and is faint/barely perceptible and within ≤10% of the invasive tumor cells. Cases as IHC 2+ were submitted to chromogenic in situ hybridization (CISH). Only IHC 2+ cases in immunohistochemistry amplified on CISH were considered positive for statistical purposes. According to Dosaka-Akita et al. [23] MGAT5 expression was classified as high (≥50% was positive) or low (<50% was negative) and lectin histochemistry staining was considered negative for <10%, moderate for 10% to 30% and intense for >30% of stained neoplastic cells. All staining score were evaluated using light microscopy.
Chromogenic in situ hybridization (CISH)
Positive (2+) immunohistochemistry HER-2 cases were also evaluated by chromogenic in situ hybridization (CISH). The ZytoDot 2C SPEC HER2/CEN 17 probe kit (Zytovision, Bremerhaven, Germany) was used for the detection of the human HER-2 gene and alpha-satellites of chromosome 17. All procedures were according to the manufacturer’s instructions which indicate that two green (HER-2) and two red (CEN 17) signals were expected in a normal interphase nucleus. HER-2 was considered amplified when the HER-2/CEN 17 ratio was ≥2 on average for 60 cells. Only cases scored as 2+ by immunohistochemistry in which the HER-2 was amplified on CISH analysis were considered positive.
Statistical analyses
Statistical analysis we carried out according to dos-Santos et al. [26], where the results were presented as positive (3+: intense positive staining and 2+: moderate positive staining) or negative (1+: weak positive staining and 0: negative staining) for tumor cells. Tests were performed with PASW Statistics 19.0 software (Chicago, IL, USA). The relationships among SNA staining, immunohistochemistry and lectin histochemistry findings and clinic-pathologic features were tested with cross tables applying the χ2 (chi-square; three or more variables) or Fisher tests (two variables), and all tests were 2-tailed. Kaplan-Meier curves, using the log-rank test, were used to estimate and compare disease-specific survival, disease-free survival (DFS) and metastasis-free survival (MFS). The independent prognostic value of α2,6-linked sialic acids expression revealed by SNA was performed using Cox Proportional Hazards Model. Results with p-value <0.05 were considered significant.
Results
Lectin histochemistry
Lectin histochemistry staining results were analyzed by χ2 (chi-square) test.
In IDC patients PHA-L staining presented a correlation with age (p=0.028; 54.5% who were older than 50 year-old were PHA-L negative; 84 out of 154), lymph node status (p=0.029; 62%, 44 patients with lymph nodes committed out of 71 patients PHA-L positive) and menopause status (p=0.015; 76.1% of post-menopausal patients were PHA-L positive; 54 out of 71).
MAL-II staining correlated to age (p=0.036; 68,6% of IDC patients who were older than 50 year-old were MAL-II positive; 48 out of 70) and menopause status (p=0.003; 78.6% of post-menopausal patients were MAL-II positive; 55 out of 70).
IDC patients who expressed α2,6-linked sialic acid had a positive relation to as follow: death (p=0.011) and lymph node status (p=0.017). All of the clinicopathologic features of prognostic significance and their respective correlations with SNA staining are shown in Table 2.
Table 2.
SNA histochemistry, clinic-histopathologic features and immunohistochemistry in IDC
| SNA (n=225) | |||||
|---|---|---|---|---|---|
|
| |||||
| Negative (n=150) | % | Positive (n=75) | % | P | |
| Age (years) | |||||
| <50 | 64 | 42.7 | 28 | 37.3 | 0.267a |
| >50 | 86 | 57.3 | 47 | 62.7 | |
| Menstrual Status | |||||
| Pre-menopausal | 57 | 38.0 | 21 | 28.0 | 0.090a |
| Post-menopausal | 93 | 62.0 | 54 | 72.0 | |
| Size (mm) | |||||
| <20 | 44 | 29.3 | 24 | 32.0 | 0.794b |
| 20-50 | 61 | 40.7 | 27 | 36.0 | |
| >50 | 45 | 30.0 | 24 | 32.0 | |
| Metastasis | 0.064a | ||||
| No | 114 | 76.0 | 49 | 65.3 | |
| Yes | 36 | 24.0 | 26 | 34.7 | |
| Death | 0.011a | ||||
| No | 112 | 74.7 | 44 | 58.7 | |
| Yes | 38 | 25.3 | 31 | 41.3 | |
| Recurrence | |||||
| No | 132 | 88.0 | 64 | 85.3 | 0.357a |
| Yes | 18 | 12.0 | 11 | 14.7 | |
| Tumor Grade | |||||
| I | 53 | 35.3 | 29 | 38.7 | 0.775b |
| II | 72 | 48.0 | 36 | 48.0 | |
| III | 25 | 16.7 | 10 | 13.3 | |
| Lymph node Status | |||||
| Negative | 80 | 53.3 | 28 | 37.3 | 0.017a |
| Positive | 70 | 46.7 | 47 | 62.7 | |
| Tumor Stage | |||||
| I | 21 | 14.0 | 11 | 14.7 | 0.932b |
| II | 69 | 46.0 | 33 | 44.0 | |
| III | 53 | 35.3 | 26 | 34.7 | |
| IV | 7 | 4.7 | 5 | 6.7 | |
| Estrogen Receptor | |||||
| Negative | 56 | 37.3 | 20 | 26.7 | 0.073a |
| Positive | 94 | 62.7 | 55 | 73.3 | |
| Progesterone Receptor | |||||
| Negative | 65 | 43.3 | 22 | 29.3 | 0.029a |
| Positive | 85 | 56.7 | 53 | 70.7 | |
| Ki-67 | |||||
| Negative | 89 | 59.3 | 54 | 72.0 | 0.042a |
| Positive | 61 | 40.7 | 21 | 28.0 | |
| p53 | |||||
| Negative | 55 | 36.7 | 21 | 28.0 | 0.125a |
| Positive | 95 | 63.3 | 54 | 72.0 | |
| HER-2 | |||||
| Negative | 121 | 80.7 | 57 | 76.0 | 0.260a |
| Positive | 29 | 19.3 | 18 | 24.0 | |
| MUC1 | |||||
| Negative | 103 | 68.7 | 36 | 48.0 | 0.002a |
| Positive | 47 | 31.3 | 39 | 52.0 | |
Fisher’s exact test;
X2 test.
IDC results showed that 33.3% were SNA positive (Figure 1A), 31.5% were PHA-L positive (Figure 1B), 31.1% were MAL-II positive. χ2 test analyzes for recognition of α2,3- and α2,6-linked sialic acids revealed that 116 (51.5%) of all IDC cases were simultaneously negative for MAL-II and SNA and 36 (16%) cases were simultaneously positive for these two lectins (p=0.0001).
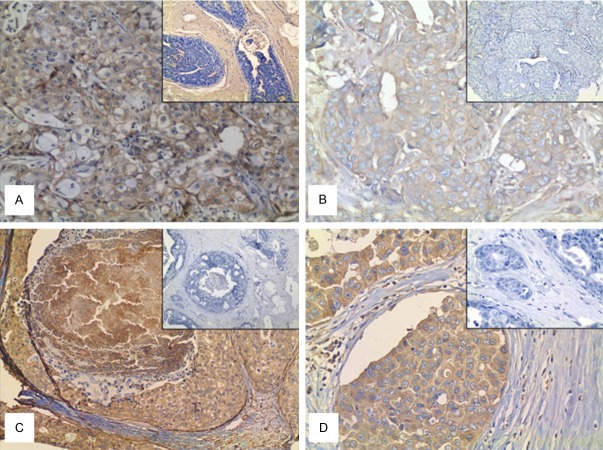
Figure 1.
Immuno- and lectin histochemistry of Invasive ductal Carcinoma (IDC) and In situ ductal Carcinoma (DCIS) of Breast. A: SNA positivity in IDC (200x). B: PHA-L positivity in IDC (200x). Details in A and B show the negative staining for SNA and PHA-L, respectively, in IDC (observe that less than 10% of the cells were stained). C: Positive case for MGAT5 in DCIS (100x). D: Positive case for PHA-L in DCIS (200x). Details in C and D show a negative control of immune- and lectin histochemistry, respectively.
Regarding DCIS cases, 51% were post-menopausal, 62.7% presented multifocal and architectural patterns as solid, comedo and cribriform. Lectin histochemistry for MAL-II, SNA and PHA-L was not correlated to any clinic-pathologic parameters (age, menstrual status, size, nuclear grade and multifocal) in DCIS samples.
Regarding the relationship between MGAT5 and PHA-L, at IDC diagnosis, 108 (48%) cases were simultaneously negative for both and 62 (27.6%) cases were simultaneously positive (p=0.0001). At DCIS cases 23.9% (16) cases were positive for MGAT5 (Figure 1C) and PHA-L (Figure 1D) with p=0.013. We also observed that 68.7% (46 cases) were simultaneously negative for SNA and MAL-II (p=0.022).
Immunohistochemistry
In IDC biopsies 108 cases (48%) were positive for MGAT5; 82 cases (36.4%) were positive for Ki67; 138 cases (61.3%) were PR positive; 149 cases (66.2%) were ER positive; 86 cases (38.2%) were positive for MUC1 and 47 cases (20.8%) positive for HER-2.
IDC MGAT5 positive samples presented correlation with MUC1 expression (p=0.044) and ER expression (p=0.046) but not with Ki67 (p=0.299), PR (p=0.205) and p53 (p=0.416).
SNA staining inversely correlated with PR positive cases (p=0.029) and Ki-67 (p=0.042) and positively related with MUC1 (p=0.002). MAL-II and PHA-L presented no correlation with the immunohistochemistry markers evaluated.
DCIS samples were MGAT5 positive in 41 cases out of 67 (61.2%), ER positive in 46 cases (68.6%); PR and HER-2 were positive in 40 cases (59.7%), each. Immunohistochemistry markers (ER, PR and HER-2) presented no correlation with MGAT5.
Analysis of the lectin histochemistry and immunohistochemistry correlation showed that 33 (49.3%) of PHA-L negative patients were HER-2 positive (p=0.006).
Survival analysis
Disease-specific Survival or overall survival (DSS), Metastasis Free Survival (MFS) and Disease-free Survival (DFS) were defined as the number of months from diagnosis to the time of death due to breast cancer, the time from diagnosis until the appearance of distant metastasis and the time from diagnosis to the occurrence of local recurrence or metastasis, respectively.
Survival rates (DSS, MFS and DFS) were analyzed regarding their correlation to immunohistochemistry for MGAT5 in IDC but no correlation was found.
Lectin histochemistry and survival rates correlation was evaluated questioning whether the expression of carbohydrates in the tumor cells can be used as a biomarker for survival analysis in IDC, in our case sialic acid residues and β1,6-N-acetilglycosamine.
Results showed that only SNA correlated with survival. It was observed that this lectin which recognizes α2,6-linked sialic acids on tumor cells correlated with DSS (Figure 2A). It was observed a significant difference (p=0.024) between the presence and the absence of this carbohydrate on cells surface. DSS decreased as patients overexpressed α2,6-linked sialic acid in neoplastic cells. Correlation is also observed for DFS (p=0.041), (Figure 2B) but not for MFS (p=0.106).
Figure 2.
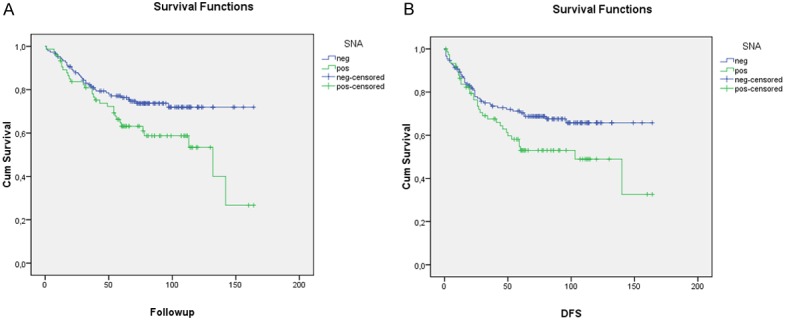
Univariate analysis (Kaplan Mayer table followed by log-rank test) of the prognostic impact of α2,6-linked sialic acids status: A: in disease-specific survival of breast cancer patients (p=0.024); B: in disease-free survival of breast cancer patients.
In Multivariate Cox Analysis, SNA was found to be an independent variable (p=0.004) able to predict the probability of survival of patients with breast cancer (Table 3 and Figure 3). In these samples the probability of survival of the patients who express this carbohydrate considerably drops with time.
Table 3.
Cox Regression Model for significant variables in the univariate analysis of overall survival
| Co-variables | p-Value | Exp (B) | 95.0% CI for Exp (B) | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age | 0.211 | 0.472 | 0.141 | 0.836 |
| Size* | 0.155 | - | 0.354 | 1.123 |
| Size (1) | 0.105 | 0.438 | 0.162 | 1.189 |
| Size (2) | 0.770 | 1.106 | 0.562 | 2.176 |
| Tumor Grade** | 0.037 | - | 0.313 | 1.561 |
| Grade (1) | 0.019 | 0.343 | 0.339 | 1.270 |
| Grade (2) | 0.382 | 0.699 | 0.066 | 3.721 |
| Limph node Status | 0.211 | 0.657 | 0.148 | 1.678 |
| Tumor Stage*** | 0.397 | - | 0.346 | 2.834 |
| Tumor Stage (1) | 0.495 | 0.496 | 0.450 | 2.248 |
| Tumor Stage (2) | 0.261 | 0.498 | 0.072 | 0.280 |
| Tumor Stage (3) | 0.986 | 0.991 | 0.381 | 1.422 |
| Recurrence | 0.989 | 1.006 | 0.797 | 2.612 |
| Metastasis | 0.000 | 0.142 | 0.759 | 2.814 |
| Menopausal Status | 0.810 | 1.158 | 0.351 | 3.826 |
| SNA | 0.004 | 0.012 | 0.001 | 0.244 |
| HER-2 | 0.358 | 0.731 | 0.005 | 1.210 |
| Ki67 | 0.361 | 0.736 | 0.593 | 2.097 |
| ER | 0.225 | 1.443 | 0.162 | 1.189 |
| PR | 0.256 | 1.462 | 0.562 | 2.176 |
| p53 | 0.736 | 1.115 | 0.351 | 3.826 |
Size (<20; 20-50; >50 mm);
Tumor Grade I, II and III;
Tumor Stage I, II, III and IV.
Figure 3.
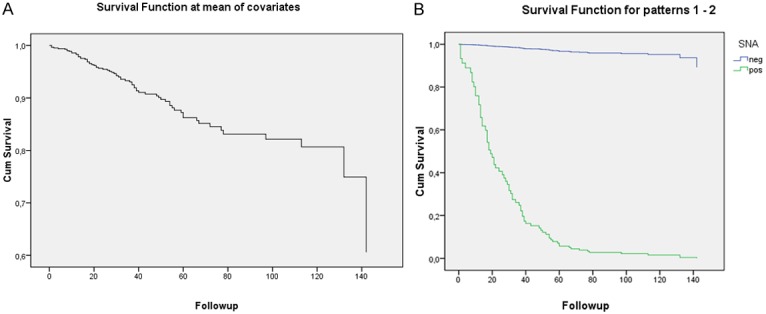
Cox-Regression for SNA staining (p=0.004). A: Follow up time regarding the survival of patients. B: Survival time of patients who overexpressed α2,6-linked sialic acids on cell surface (green curve) and lack this aberrant expression (blue curve).
DCI versus DCIS
The χ2 (chi-square) test was performed for lectin histochemistry and immunohistochemistry findings in patients with IDC and DCIS to compare the parameters in both groups. Correlation was carried out for MGAT5, SNA, MAL-II, PHA-L, ER and PR. Results showed that, once more, only SNA staining was significant (p=0.034) indicating a higher content of α2,6 sialic acids in IDC and a lower in DCIS.
Discussion
In this study the SNA staining (evaluated by lectin histochemistry) and its relationship with several cell biomarkers, including MGAT5, MUC1 and laboratory routine markers (ER, PR, HER-2, Ki67 and p53) were analyzed in a tissue microarray (TMA) cohort of 225 patients with invasive ductal carcinoma (IDC) of breast. Our study focused on evaluates the relationship between the expression of tumor-associated carbohydrates and well-established prognostic factors in patients with IDC and compare to ductal carcinoma in situ (DCIS) to assay the differences between pre-malign and malign lesions. TMA was a trustful technique to access the protein expression and the results from its application are significant and reproducible as observed by several other authors who also used this technique to validate biomarkers [26,32].
It is known that changes in glycosylation of glycoproteins or glycolipids often cause a change in their function and such phenomenon is correlated with cancerous transformation [33,34]. According to Cui et al. [35] the overall survival of the patients who express different levels of α2,3-sialic acid residues considerably drops and the occurrence of changes in the glycocode of these glycoconjugates of cell surface stimulating/modulating adhesion, migration and invasion of breast cancers cells. The authors used 50 primary tumor samples, 50 pair-matched lymph node metastasis tumor samples and MDA-MB-231, T-47D and MCF-7 breast cancer cell lines with different metastatic potential and they concluded that lymph node metastasis tumor samples exhibited higher levels of expression of α2,3-sialic acid residues compared to that of primary tumors. Differently, our study was designed with IDC samples (225) and we found that the overexpression of the α2,6-sialic acid residues, not α2,3, is closely related with the decreased survival of patients, which is in accordance with others types of cancer, including breast [12,36,37].
Breast cancer development and progression are regulated by estrogen and its receptor (ER) along others predictive markers such as progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2) [38]. Our study showed that 94 (41.8%) patients positive to ER and 85 (37.8%) patients positive to PR were negative to SNA staining with PR negatively correlating with the expression α2,6-linked sialic acid (p=0.029). Semaan et al. [38] studied the glycoproteins in human breast cancer ER+ tissues and in ER- tissues and found that some proteins were more glycosylated at the ER- tissues. It is known that the loss of the hormonal receptor has been correlated with a more aggressive stage of the disease and this loss may affect the levels of sialic acids on tumor cells to be confirmed after in vitro and in vivo studies by our group.
MUC1 is usually present on the apical surface of secretory epithelia in normal cells and in malignant tissues, when cells lose their polarity, MUC1 is found both in cytoplasm and membrane, where there is an expression up to 90% of cases. This overexpression leads to the loss of cell-cell and cell-extracellular matrix adhesion and, therefore, promotes cancer metastasis [30,39,40]. In our study MUC1 was correlated with α2,6-sialic acid (p=0.002). Cancer cells surface MUC1 was observed to be abnormally glycosylated presenting in its structure many linkage sites to receive sialic acids [41-43].
Lectins have been a target of research in cancer glycobiology and have been used as a probe to investigate the cellular glycocode because they recognize carbohydrates in a specific way [19]. These molecules have been used in histopathology investigation for mapping stages of differentiation, levels of malignancy and metastatic ability in human brain tissue [20], breast [44-47], non-melanoma skin cancer [48] and other tissues [23,49].
PHA-L binding profile and GnT-V overexpression (gene that encodes the enzyme MGAT5) has been associated with invasive/metastatic potential of cancer cells and consequent poor prognosis in several human carcinomas [13,17,23,50]. However, according to Huang et al. [51] MGAT5 has been demonstrated to display a distinct function in different types of tumors; down-regulation of GnT-V decreased proliferation and the metastatic/invasive potential of BGC823 gastric cancer cells in vitro). Dosaka-Akita et al. [23], used 217 surgically resected NSCLCs to assay the activity of GnT-V by PHA-L histochemistry and after survival analysis concluded that GnT-V low expression associated with shorter survival and poor prognosis in non-small cell lung cancers. Handerson et al. [17], used a TMA with more than 400 patients with breast cancer and viewed that the abundance of β1,6-branched oligosaccharides (via PHA-L histochemistry) was directly associated with breast carcinoma nodal metastasis. In our study MGAT5 was overexpressed in 48% of IDC samples and do not correlated with survival. It is important to highlight that studies with breast cancer [17,52] were conducted with PHA-L histochemistry alone, not with MGAT5 immunohistochemistry combined with PHA-L histochemistry like ours. Our study is the first regarding invasive and in situ ductal carcinoma addressing immuno- and histochemistry with MGAT5 and PHA-L, respectively, and although the related findings with these markers and the results showed a significant biologic importance of β1,6-branched oligosaccharides for breast cancer progression, the prognostic effect of PHA-L and MGAT5 is not strong enough to add independent information to the established clinicopathologic variables.
We also found that the overexpression of α2,6-linked sialic acid (recognized by SNA) can be a predictor of overall survival and disease-free survival; survival of patients who express this carbohydrate considerably drops with time. These findings are in accordance with some authors who described that the sialic acid profile on the tumor cell surface is an indicative of a poor prognosis in colon, ovarian and breast cancer [53-55]. Ferreira et al. [48], investigated the expression of α2,6 and α2,3-linked sialic acids on non-melanoma skin cancer using SNA and MAL-II lectins histochemistry and found that these lectins can differentiate the stages of pre-malign and malign tumors. Other study was conducted by Borzym-Kluczyk and Radziejewska [56] in renal cancer tissues using Elisa-test with SNA and MAL-II, the authors founded that secreted α2,6 and α2,3-linked sialic acids were significantly increased by cancerous cells when compared to normal and intermediate renal tissue. All these findings are important to show that lectins can be use as prognostic probes to recognize the differences in carbohydrates expression pattern in pre-malign and malign lesions.
It is known that high expression of sialic acids promotes a series of damages on cells, leading to a series of steps that can culminate with metastasis. MAL-II lectin strongly binds to α2,3-linked sialic acid residues [57] but this lectin did not show correlation with any survival parameters. In our study MAL-II was positive in 70 samples (31.1%), not statistically different to SNA (75 samples; 33.3%) but it is worth to note that most patients who died or presented local recurrence or metastasis had the expression of α2,6-linked sialic acid affecting the survival curves. Besides we observed that the α2,6-sialylation was associated with poor prognosis in tumorigenesis as also observed by Swindall and Bellis in colon carcinoma cells [58].
In breast cancers the most expressed glycosyltransferase is ST3Gal III which catalyzes the transfer of α2,3-linked sialic acid residues to terminal galactose (Gal) residues located on either Gal-β3GlcNAc or Galβ1-4GlcNAc structures. This enzyme has positive correlation with tumor size and lymph node status; moreover high ST3Gal III/ST6Gal I ratio (ST6Gal I which mediates the transfer of sialic acid with a α2,6-linkage to terminal Gal) is correlated with a shorter overall survival and poor prognosis [58-60]. In addition, ST6GalNAc V (which mediates the transfer of sialic acid with a α2,6-linkage to GalNAc residues) has been reported to mediate brain metastasis of breast cancer cells [61]. All these enzymes add sialic acids on the terminal portion of glycoproteins and can be altered in breast cancers, which are targets of studies in our group.
Our results showed that most of DCIS patients were SNA negative, completely different of IDC where the expression of this carbohydrate is associated with the survival of the patients. The sialic acids profile shows a relevant difference between DCIS and IDC group (p=0.034), as we observed using SNA, that turned out to be lower in DCIS patients compared IDC ones. It is important to note that the comparison between IDC and DCIS tumors showed no correlation regarding MGAT5 or other marker expressed in these two groups of patients. It is known that DCIS is considered a premalignant stage of IDC with great differences comparing to normal tissues and the presence of α2,6 sialic acids residues in glycoconjugates of tumor cell surface can be a indicative of a worse prognostic to patients diagnosed with IDC [62].
Although DCIS being considered as a precursor lesion for IDC [62,63], the reported incidence of accompanying DCIS in IDC is not fully understood. Our results showed a clear difference regarding SNA histochemistry profile in IDC samples and DCIS, more importantly these differences can be easily assayed by simples and efficient techniques.
Conclusions
Exploitation of difference in cell surface glycans between invasive ductal and in situ ductal carcinoma cells provides opportunities to discover new biomarkers to personalize diagnosis and treatment of cancer based in the fact that the combination of several biomarkers may lead to better prognosis information. Our findings showed that SNA staining pattern strongly suggests that this lectin can be used as an easy probe to access sialic acid residues profile and then assist in patient prognosis. The present study also contributes to future researches for the potential of these biomarkers as putative therapeutic target for breast carcinomas.
Acknowledgements
We thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) and FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil) for financial support, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) and FACEPE (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco, Brazil) for schollarships and financial support; and Deisy Mara da Silva for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics. Ca Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Cancer. Estimate 2014: incidence of cancer in Brazil. Available from: http://www.inca.org.br. Accessed 20 Jan 2014.
- 3.Bertucci F, Finetti P, Roche H, Le Doussal JM, Marisa L. Comparison of the prognostic value of genomic grade index, Ki67 expression and mitotic activity index in early node-positive breast cancer patients. Ann Oncol. 2013;24:625–632. doi: 10.1093/annonc/mds510. [DOI] [PubMed] [Google Scholar]
- 4.Kuzmanov U, Kosanam H, Diamandis EP. The sweet and sour of serological glycoprotein tumor biomarker quantification. BMC Med. 2013;11:31. doi: 10.1186/1741-7015-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake PM, Cho W, Li B, Prakobphol A, Johansen E, Anderson NL, Regnier FE, Gibson BW, Fisher SJ. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin Chem. 2010;56:223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99:10231–1023. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochim Biophys Acta. 2012;1820:1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Varki A, Lowe JB. Biological Roles of Glycans. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 6. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1897/ [Google Scholar]
- 9.Taniguchi N, Korekane H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB Rep. 2011;44:772–781. doi: 10.5483/bmbrep.2011.44.12.772. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Fukuda M. Cell type-specific roles of carbohydrates in tumor metastasis. Methods Enzymol. 2006;416:371–380. doi: 10.1016/S0076-6879(06)16024-3. [DOI] [PubMed] [Google Scholar]
- 11.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund M, Ng E, Varki A, Varki NM. Alpha 2-6-linked sialic acids on n-glycans modulate carcinoma differentiation in vivo . Cancer Res. 2012;68:388–394. doi: 10.1158/0008-5472.CAN-07-1340. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira JT, de Matos AJ, Santos AL, Pinto R, Gomes J, Hespanhol V, Chammas R, Manninen A, Bernardes ES, Albuquerque Reis C, Rutteman G, Gärtner F. Sialylation regulates galectin-3/ligand interplay during mammary tumour progression-a case of targeted uncloaking. Int J Dev Biol. 2011;55:823–834. doi: 10.1387/ijdb.113359jt. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Kim S, Kang J, Ko J. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep. 2012;45:623–628. doi: 10.5483/BMBRep.2012.45.11.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui SF, Pawelek J, Handerson T, Lin CY, Dickson RB, Rimm DL, Camp RL. Coexpression of beta1,6-N-acetylglucosaminyltransferase V glycoprotein substrates defines aggressive breast cancers with poor outcome. Cancer Epidemiol Biomarkers Prev. 2005;14:2517–2523. doi: 10.1158/1055-9965.EPI-05-0464. [DOI] [PubMed] [Google Scholar]
- 16.Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S, Honda H, Takemura F, Taniguchi N, Imaoka S. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res. 2000;6:1772–1777. [PubMed] [Google Scholar]
- 17.Handerson T, Camp R, Harigopal M, Rimm D, Pawelek J. Beta1,6-branched oligosaccharides are increased in lymph node metastases and predict poor outcome in breast carcinoma. Clin Cancer Res. 2005;11:2969–2973. doi: 10.1158/1078-0432.CCR-04-2211. [DOI] [PubMed] [Google Scholar]
- 18.Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982;257:11230–11234. [PubMed] [Google Scholar]
- 19.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 20.Beltrão EIC, Medeiros PL, Rodrigues OG, Figueredo-Silva J, Valença MM, Coelho LCBB, Carvalho LB. Parkia pendula lectin as histochemistry marker for meninge tumor. Eur J Histochem. 2003;47:139–142. doi: 10.4081/819. [DOI] [PubMed] [Google Scholar]
- 21.Sobral APV, Rêgo MJBM, Cavalacanti CL, Carvalho LB Jr, Beltrão EI. ConA and UEA-I lecin histochemistry of parotid gland mucoepidermoid carcinoma. J Oral Sci. 2010;52:49–54. doi: 10.2334/josnusd.52.49. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira AS, Vasconcelos JLA, Silva RCWC, Cavalcanti CLB, Rêgo MJBM, Beltrão EIC. Expression Patterns of á2,3-sialyltransferase I and á2,6-sialyltransferase I in human cutaneous epithelial lesions. Eur J Histochem. 2013;57:41–5. doi: 10.4081/ejh.2013.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh T, Katoh H, Taniguchi N. Expression of N-acetylglucosaminyltransferase v is associated with prognosis and histology in non-small cell lung cancers. Clin Cancer Res. 2004;1:1773–79. doi: 10.1158/1078-0432.ccr-1047-3. [DOI] [PubMed] [Google Scholar]
- 24.López-Morales D, Reyes-Leyva J, Santos-López G, Zenteno E, Vallejo-Ruiz V. Increased expression of sialic acid in cervical biopsies with squamous intraepithelial lesions. Diagn Pathol. 2010;5:1–5. doi: 10.1186/1746-1596-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro-Silva A, Moutinho MAH, Moura B, Vale FR, Zucoloto S. Expression of checkpoint kinase 2 in breast carcinomas: correlation with key regulators of tumor cell proliferation, angiogenesis, and survival. Histol Histopathol. 2006;21:373–82. doi: 10.14670/HH-21.373. [DOI] [PubMed] [Google Scholar]
- 26.dos Santos PB, Zanetti JS, Ribeiro-Silva A, Beltrão EIC. Beta 1 integrin predicts survival in breast cancer: a clinicopathological and immunohistochemical study. Diagn Pathol. 2012;16:104–115. doi: 10.1186/1746-1596-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fountzilas G, Dafni U, Bobos M, Batistatou A, Kotoula V, Trihia H, Malamou-Mitsi V, Miliaras S, Chrisafi S, Papadopoulos S, Sotiropoulou M, Filippidis T, Gogas H, Koletsa T, Bafaloukos D, Televantou D, Kalogeras KT, Pectasides D, Skarlos DV, Koutras A, Dimopoulos MA. Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel. PLoS One. 2012;7:e37946. doi: 10.1371/journal.pone.0037946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badye S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ercan C, Van Diest P, Van Der Ende B, Hinrichs J, Bult P, Buerguer H, van der Wall E, Derken PWB. p53 mutations in classic and pleomorphic invasive lobular carcinoma of the breast. Cell Oncol (Dordr) 2012;35:111–118. doi: 10.1007/s13402-012-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanetti JS, Soave DF, Oliveira-Costa JP, da-Silveira GG, Ramalho LN, Garcia SB, Zucoloto S, Ribeiro-Silva A. The role of tumor hypoxia in MUC1-positive breast carcinomas. Virchows Arch. 2011;459:367–375. doi: 10.1007/s00428-011-1142-6. [DOI] [PubMed] [Google Scholar]
- 31.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 32.Lawson MH, Cummings NM, Rassl DM, Vowler SL, Wickens M, Howat WJ, Brenton JD, Murphy G, Rintoul RC. Bcl-2 and β1-integrin predict survival in a tissue microarray of small cell lung cancer. Br J Cancer. 2010;103:1710–1715. doi: 10.1038/sj.bjc.6605950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Y, Zhang H. Characterization of disease-associated N-linked glycoproteins. Proteomics. 2013;13:504–11. doi: 10.1002/pmic.201200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–69. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 35.Cui H, Lin Y, Yue L, Zhao X, Liu J. Differential expression of the α2,3-sialic acid residuesin breast cancer is associated with metastatic potential. Oncol Rep. 2011;25:1365–1371. doi: 10.3892/or.2011.1192. [DOI] [PubMed] [Google Scholar]
- 36.Lise M, Belluco C, Perera SP, Patel R, Thomas P, Ganguly A. Clinical correlations of a2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma. 2000;19:281–286. doi: 10.1089/027245700429828. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Kemmner W, Grigull S, Schlag PM. Cell surface a2,6 sialylation affects adhesion of breast carcinoma cells. Exp Cell Res. 2002;276:101–110. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- 38.Semaan SM, Wang X, Marshall AG, Sang QXA. Identification of potential glycoprotein biomarkers in estrogen receptor positive (ER+) and negative (ER-) human breast cancer tissues by LC-LTQ/FT-ICR mass spectrometry. J Cancer. 2012;3:269–284. doi: 10.7150/jca.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran DT, Ten Hagen KG. Mucin-type O-glycosylation during development. J Biol Chem. 2013;288:6921–6929. doi: 10.1074/jbc.R112.418558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res. 2006;8:R37. doi: 10.1186/bcr1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J. A Core protein epitope of the polymorphic epithelial mucin detected by the monoclonal-antibody sm-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989;43:1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 42.Horm TM, Schroeder JA. MUC1 and metastatic cancer: Expression, function and therapeutic targeting. Cell Adh Migr. 2010;7:1–12. doi: 10.4161/cam.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai J, Hattori N, Nakajima M, Moriya T, Suzuki T, Yokoyama A, Kohno N. Differential expression of the glycosylated forms of MUC1 during lung development. Eur J Histochem. 2007;51:95–102. [PubMed] [Google Scholar]
- 44.Beltrão EIC, Correia MT, Figueredo-Silva J, Coelho LC. Binding evaluation of isoform Cratylia mollis lectin to human mammary tissues. Appl Biochem Biotechnol. 1998;74:125–134. doi: 10.1007/BF02825961. [DOI] [PubMed] [Google Scholar]
- 45.Korourian S, Siegel E, Kieber-Emmons T, Monzavi-Karbassi B. Expression analysis of carbohydrate antigens in ductal carcinoma in situ of the breast by lectin histochemistry. BMC Cancer. 2008;8:136. doi: 10.1186/1471-2407-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rêgo MJBM, Vieira-de-Mello GS, Santos CAS, Chammas R, Beltrão EIC. Implications on glycobiological aspects of tumor hypoxia in breast ductal carcinoma in situ. Med Mol Morphol. 2013;46:92–96. doi: 10.1007/s00795-013-0013-4. [DOI] [PubMed] [Google Scholar]
- 47.Vieira-de-Mello GS, Silva-Filho AF, Barros PS, Rêgo MJBM, Beltrão EIC. Lectin histochemistry reveals changes in carbohydrate expression on morphological types of breast ductal carcinoma in situ. J Cytol Histol. 2013;4:1–4. [Google Scholar]
- 48.Ferreira SA, Vasconcelos JLA, Cavalcanti CLB, Rêgo MJMB, Beltrao EIC. Sialic Acid differential expression in non-melanoma skin cancer biopsies. Med Mol Morphol. 2013;46:198–202. doi: 10.1007/s00795-013-0025-0. [DOI] [PubMed] [Google Scholar]
- 49.Lima LR, Bezerra MF, Almeida SM, Silva LP, Beltrão EIC, Carvalho Júnior LB. Glycophenotype evaluation in cutaneous tumors using lectins labeled with acridinium ester. Dis Markers. 2013;35:149–154. doi: 10.1155/2013/787130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Kang JG, Song KJ, Jeon SK, Oh S. N-Acetylglucosaminyltransferase V triggers overexpression of MT1-MMP and reinforces the invasive/metastatic potential of cancer cells. Biochem Biophys Res Commun. 2013;431:658–663. doi: 10.1016/j.bbrc.2013.01.065. [DOI] [PubMed] [Google Scholar]
- 51.Huang B, Wu Q, Ge Y, Zhang J, Sun L, Zhang Y, Fu L, Fan J, Wang Z. Expression of N-acetylglucosaminyltransferase V in gastric cancer correlates with metastasis and prognosis. Int J Oncol. 2014;44:849–857. doi: 10.3892/ijo.2014.2248. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW. Beta 1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res. 1991;51:718–723. [PubMed] [Google Scholar]
- 53.Bresalier RS, Byrd JC, Wang L, Raz A. Colon cancer mucin: a new ligand for the beta-galactoside-binding protein galectin-3. Cancer Res. 1996;56:4354–4357. [PubMed] [Google Scholar]
- 54.Dube DH, Bertozzi CR. Glycans in cancer and inflammation - potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 55.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borzym-Kluczyk M, Radziejewska I. Changes of the expression of Lewis blood group antigens in glycoproteins of renal cancer tissues. Acta Biochim Pol. 2013;60:223–226. [PubMed] [Google Scholar]
- 57.Imberty A, Gautier C, Lescar J, Perez S, Wyns L, Loris R. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J Biol Chem. 2000;275:17541–17548. doi: 10.1074/jbc.M000560200. [DOI] [PubMed] [Google Scholar]
- 58.Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286:22982–22990. doi: 10.1074/jbc.M110.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Recchi MA, Hebbar M, Hornez L, Harduin-Lepers A, Peyrat JP, Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58:4066–4070. [PubMed] [Google Scholar]
- 60.Hebbar M, Krzewinski-Recchi MA, Hornez L, Verdiere A, Harduin-Lepers A, Bonneterre J, Delannoy P, Peyrat JP. Prognostic value of tumoral sialyltransferase expression and circulating E-selectin concentrations in node-negative breast cancer patients. Int J Biol Markers. 2003;18:116–122. doi: 10.1177/172460080301800204. [DOI] [PubMed] [Google Scholar]
- 61.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cakir A, Gonul II, Uluoglu O. A comprehensive morphological study for basal-like breast carcinomas with comparison to nonbasal-like carcinomas. Diagn Pathol. 2012;7:1–11. doi: 10.1186/1746-1596-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devilee P, Tavassoli FA. World Health Organization: Tumours of the breast and female genital organs. Oxford [Oxfordshire]: Oxford University Press; 2003. [Google Scholar]