Abstract
Diosmetin (3’, 5, 7-trihydroxy-4’-methoxyflavone), the aglycone part of the flavonoid glycosides diosmin occurs naturally in citrus fruit, was considered to exhibit anti-inflammatory and antioxidant properties. Our study aimed to investigate the effect of diosmetin in a murine model of cerulein-induced acute pancreatitis (AP). Experimental AP was induced in mice by seven intraperitoneal injection of cerulein (50 ug/kg) at hourly intervals. Diosmetin (100 mg/kg) or vehicle was pretreated 2 h before the first cerulein injection. After 6 h, 9 h, 12 h of the first cerulein injection, the severity of acute pancreatitis was evaluated biochemically and morphologically. Pretreatment with diosmetin significantly reduced serum levels of amylase and lipase; the histological injury; the secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6; myeloperoxidase (MPO) activity, trypsinogen activation peptide (TAP) level, the expression of inducible nitric oxide synthase (iNOS); and the nuclear factor (NF)-κB activation in cerulein-induced AP. This study showed that administration of diosmetin demonstrated a beneficial effect on the course of cerulein-induced AP in mice. Therefore, diosmetin may become a new therapeutic agent in future clinical trials for treatment of AP.
Keywords: Acute pancreatitis, diosmetin, inflammation, iNOS, cytokine, NF-κB
Introduction
AP is a potentially lethal inflammatory disease with a wide spectrum of clinical presentations ranging from a mild self-limited condition after supportive therapy to a life-threatening disease with high incidence of complications and high mortality [1,2]. Some 10-20% of these die of multiorgan failure despite intense experimental and clinical tests of potential drugs treatment or surgery, which is in part due to insufficiency of available pharmacologic options for treating AP [3,4]. It is well-recognized that trypsinogen activation, inflammation, oxidative stress and activation of nuclear factor (NF)-κB are important pathophysiological components in AP [2,5,6]. Intra-acinar cell activation of digestive enzymes such as trypsinogen is thought to be the triggering event of the disease, resulting in inflammation and acinar cell death [7]. During AP, acinar cells synthesize and release inflammatory mediators, such as cytokines and chemokines, resulting in recruitment of inflammatory cells such as neutrophils and macrophages, leading to further acinar cell injury and inducing a cascade of various proinflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [8-10]. Imbalance between endogenous oxygen-free radical scavenger pathways and mechanisms for generation of these free radicals is one of the forerunners of AP [5,11].
Diosmetin (3’, 5, 7-trihydroxy-4’-methoxyflavone) is the aglycone of the flavonoid glycoside diosmin (3’, 5, 7-trihydroxy-4’-methoxyflavone-7-ramnoglucoside) which occurs naturally in the legume Acacia farnesiana Wild and Olea europaea L. leaves [12]. Diosmin is hydrolyzed to its aglycone diosmetin by intestinal micreflora enzymes before its absorption into the body [13]. Pharmacologically, it has been established that diosmetin possesses different medicinal properties such as anticancer, antimicrobial, antioxidant and anti-inflammatory activities [14-17].
Although the physiological and protective effects of diosmetin related to its potent anti-inflammatory and antioxidant activity have documented earlier in many inflammatory diseases, but its effect in AP has not been evaluated before. Here, we investigated the effects of diosmetin in a murine model of cerulein-induced AP.
Materials and methods
Ethics statement
All the animal related procedures were approved by the Animal Care and Use Committee of The Tenth People’s Hospital of Shanghai, Tongji University. Permit number: 2011-RES1. This study was also approved by Science and Technology Commission of Shanghai Municipality (ID: SYXK 2007-0006).
Animal and materials
Male C57BL/6 mice (age 8 weeks, weight 25-27 g) were purchased from Shanghai Laboratory Animal Co Ltd (SLAC, Shanghai, China). Animals were maintained on a 12 h light/12 h dark cycle at 22°C, given water ad libitum, fed standard laboratory chow and allowed to acclimatize for minimum of 1 week. Mice were randomly assigned to control or experimental groups. All the animal-related procedures were approved by the Animal Ethical Committee of Tongji University. Purified diosmetin (> 98%) was purchased from the National Institute for Control Pharmaceutical and Biological Products (Beijing, China). Cerulein, dimethyl sulfoxide (DMSO), eosin and hematoxylin were purchased from Sigma Chemical (Sigma-Aldrich, St. Louis, MO). Antibodies against NF-κB p65, inducible NO synthase (iNOS), Histone-H1 and β-actin were purchased from Abcam (Hong Kong). Peroxidase-conjugated secondary antibody was purchased from Santa Cruz Biotechnology Company (Santa Cruz, CA, USA).
Experimental design
Diosmetin was dissolved in vehicle (2% DMSO). Then three doses (25 mg/kg, 50 mg/kg, 100 mg/kg) were used to pretreat cerulein-induced AP. AP was induced by seven injections of cerulein (50 ug/kg, i.p. at intervals of 1 h) as described previously [18]. The normal control mice were given saline (0.9% NaCl) solution intraperitoneally instead of cerulein (n=8 for each group). Vehicle or diosmetin (p.o.) was administered 2 h before the first cerulein injection. All animals were sacrificed at 12 h after the first injection of cerulein, a time point at which pancreatic damage had already peaked. The effect of diosmetin was evaluated by the level of serum amylase, an indicator which was usually considered to be closely related to pancreatic damage, to get an optimal dose. The optimal dose of diosmetin (100 mg/kg) was used for the next series of experiment. Then 72 mice were divided into three groups randomly: group 1, normal control; group 2, cerulein + vehicle-treated; group 3, cerulein + diosmetin-treated. The induction of AP and administration of diosmetin or vehicle were performed the same as the preliminary study. Mice were sacrificed at 6 h, 9 h and 12 h after the first cerulein injection, 8 mice at every time point in each group. Blood samples were taken to determine the serum amylase, lipase and cytokine levels. A portion of the tail of the pancreas was fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 12 h, embedded in paraffin, and cut into 5-μm thick sections which were stained with hematoxylin and eosin to observe the morphological changes under a light microscope by standard procedures. The rest portion of each pancreas was stored at -80°C for further investigation.
Serum amylase and lipase assay
The serum activities of amylase and lipase were measured by enzyme dynamics chemistry using commercial kits according to the manufacturer’s protocols in a Roche/Hitachi modular analytics system (Roche, Mannheim, Germany).
Trypsinogen activation peptide (TAP) level
Enzyme-linked immunosorbent assay (ELISA) was used to determine concentrations of TAP of pancreas homogenates. Homogenates were prepared by homogenizing pancreatic tissue in HEPES buffer (20 mM, pH 7.4) supplemented with EDTA (1.5 mM) and a cocktail of protease inhibitors (1:100 dilution). Commercially available ELISA kits were used according to the manufacturer’s instructions (Xinqidi biological technology Co., Ltd, Wuhan, China). The TAP level was expressed in micrograms per gram of tissue.
Determination of serum proinflammatory cytokines
Blood samples of each time point were centrifuged at 3000 g for 10 min at 4°C. Serum TNF-α, IL-β and IL-6 were measured in enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Quantikine, R&D Systems, Minneapolis, MN, USA).
Myeloperoxidase (MPO) assay
Neutrophil sequestration in the pancreas was quantified by measuring tissue MPO activity according to a previously described method. The tissue samples were homogenized in 20 mM phosphate buffer (PH 7.4), and centrifuged (12,000 × g for 10 min at 4°C). The pellet was resuspended in 50 mM phosphate buffer (PH 6.0), containing 0.5% hevade cyltrimethylammonium bromide (HETAB). The suspension was subjected to four cycles of freezing and thawing and was further disrupted by sonication for 1 min. The sample was then centrifuged (12,000 × g for 5 min at 4°C). Aliquots of supernatant were added to the reaction mixture containing 0.167 mg/ml of o-dianisidine dihydrochloride and 0.0005% H2O2 solution, which were prepared in 50 mM of phosphate buffer. The change in absorbance at 450 nm was then measured for 5 min using a Beckman spectrophotometer (Beckman DU 640B, CA, USA). One unit of MPO activity was defined as that degrading 1 mmol of peroxide per min at 25°C. The activity was expressed in units per milligram of tissue.
Western blot analysis
For Western blot analysis, rat or mouse pancreas was rapidly ground in liquid nitrogen. The resulting powder was reconstituted in ice-cold RIPA buffer containing 1 mmol/l phenylmethanesulfonyl fluoride (PMSF) and a cocktail of protease inhibitors (1:100 dilution). Samples were centrifuged at 4°C for 15 min at 10,000 × g. Supernatants were recovered, and total protein was determined using the BCA method (Pierce, Rockford, LA, USA). A 50 μg portion of protein or equal proportion concentrated supernatant was subjected to sodium dodecyl sulfate/ polyacrylamide gel electrophoresis (SDS-PAGE), and then blotted following standard methods. Non-specific binding to the membrane was blocked by 5% (w/v) dry non-fat milk in Tris-buffered saline/0.05% Tween-20 (TBST) at room temperature for 1 h in a covered container. Blots were incubated overnight at 4°C with rabbit polyclonal anti-inducible NO synthase (iNOS) antibody (1:400 dilution) or anti-NF-κB p65 (1:200 dilution) diluted in 5% BSA. Membranes were washed with TBST and incubated with a secondary rabbit anti-mouse IgG horseradish peroxidase (HRP) antibody (1:2,000 dilution) diluted in 5% (w/v) dry nonfat milk in TBST for 1 h at room temperature. Finally, membranes were washed with TBST, developed using the ECL detection system (Santa Cruz Biotechnology), quickly dried, and exposed to ECL film.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples were cut into a thickness of 5 μm. Each tissue section was deparaffinized and rehydrated with graded ethanol. For antigen retrieval, slides were boiled in EDTA (1 mM, pH 8.0) for 15 min in a microwave oven. Endogenous peroxidase activity was blocked with a 0.3% hydrogen peroxide solution for 10 min at room temperature. After rinsing with PBS, slides were incubated overnight at 4°C with anti-NF-κB p65 (1:200 dilution) diluted in 5% BSA. The antibody binding was detected with an Envision Detection Kit, Peroxidase/DAB, Rabbit/Mouse (Gene Tech, Shanghai, China). Sections were counterstained with hematoxylin. For negative control, isotype-matched primary antibody replaced the antibody against NF-κB p65 followed by the same secondary antibody used in the other sections. Positive areas stained with NF-κB p65 were examined in all specimens using a microscope (CTR 6000; Leica, Wetzlar, Germany).
Statistical analysis
All results were expressed as mean ± standard deviation (SD). Statistical analysis was done using Student’s t-test for comparison of two groups, and ANOVA was used for multiple comparisons. In both cases, differences of P < 0.05 were considered statistically significant.
Results
Effect of diosmetin on pancreas pathology in cerulein induced AP
In the preliminary study, the high dose (100 mg/kg) showed more effective decrease of serum amylase and lipase compared with moderate dose (50 mg/kg) and low dose (25 mg/kg) at the time point of 12 h after the first cerulein injection which pancreatic damage had already peaked (Figure 1A and 1B). Therefore, the dose of 100 mg/kg was selected as the optimal dose for the next series of experiments. To examine the effect of diosmetin on the development and severity of AP, mice were pretreated with vehicle or diosmetin (100 mg/kg) as described in experimental design. Diosmetin significantly protected the pancreas from histological damage induced by cerulein as observed by hematoxylin and eosin staining (Figure 1C). Pretreatment with diosmetin markedly reduced the cerulein-induced histological features of pancreatic injury, characterized by lower interstitial edema, less inflammatory cell infiltration, and alleviated acinar cell necrosis.
Figure 1.
Effect of diosmetin on pancreas pathology in cerulein induced AP. Mice (n=8 for each group) were given seven hourly injection of cerulein (50 ug/kg). Diosmetin (25 mg/kg, 50 mg/kg, 100 mg/kg; p.o.) or vehicle (p.o.) was administrated 2 h before the first cerulein injection. The control group was given saline (0.9% NaCl) solution intraperitoneally instead cerulein. Mice were sacrificed 12 h after the first injection of cerulein. Changes in serum amylase level (A) and lipase level (B). Data are represented as mean ± SD. #P < 0.05 vs control group, $P < 0.05 vs vehicle-treated group, &P < 0.05 vs 25 mg/kg group, *P < 0.05 vs 50 mg/kg group. (C) Effect of diosmetin on pancreas histology in cerulein-induced AP. Mice (n=24 for each group) were given seven hourly injection of cerulein (50 ug/kg). Diosmetin (100 mg/kg; p.o.) or vehicle (p.o.) was administrated 2 h before the first cerulein injection. The control group was given saline (0.9% NaCl) solution intraperitoneally instead cerulein. Mice were sacrificed 6 h, 9 h, 12 h after the first injection of cerulein, five mice at every time point in each group. Representative hematoxylin and eosin-stained sections of pancreas are shown. Original magnifications: 200×.
Effect of diosmetin on AP-induced enzyme production and trypsinogen activation
Serum amylase and lipase are most commonly obtained as biochemical markers of pancreatic disease, particularly AP. Therefore, we assessed the severity of AP by measuring enzyme production. As shown in Figure 2A and 2B, diosmetin reduced the levels of amylase and lipase in serum significantly. TAP is used as a marker of trypsinogen activation reaction where TAP is cleaved off and trypsinogen is activated to trypsin. Pretreatment with diosmetin markedly reduced cerulein-induced TAP level in pancreas tissue (Figure 2C).
Figure 2.
Effect of diosmetin on AP-induced enzyme production and trypsinogen activation. Changes in serum amylase level (A) and lipase level (B), and pancreatic tissue TAP level (C) in all groups. Data are represented as m ean ± SD. #P < 0.05 vs controlgroup at the same time point, $P < 0.05 vs vehicle-treated group at the same time point.
Anti-inflammatory effect of diosmetin in cerulein-induced AP
During AP, the activation of NF-κB enhanced the release of many proinflammatory cytokines such as TNF-α, IL-β and IL-6. The production of these inflammatory cytokines in serum was reduced by diosmetin treatment (Figure 3A-C). Neutrophil sequestration in pancreas was quantitated by measuring tissue MPO activity, and diosmetin reduced cerulein-induced activity of MPO in pancreas tissue (Figure 3D).
Figure 3.

Anti-inflammatory effect of diosmetin in cerulein-induced AP. Serum proinflammatory cytokine such as TNF-α, IL-β and IL-6 were measured by ELISA (A-C). Changes in pancreatic tissue MPO activity (D). Data are represented as mean ± SD. #P < 0.05 vs control group at the same time point, $P < 0.05 vs vehicle-treated group at the same time point.
Antioxidant effect of diosmetin in caerulein-induced AP
The correlation between AP and oxidative-nitrosative stress has been documented in pancreatic tissue by generation of reactive oxygen species and accumulation of products of reactive oxygen species-mediated lipid peroxidation [19]. Inducible nitric oxide synthase (iNOS) is a marker of oxidative-nitrosative stress and is involved in the stimulation of inflammation. The level of iNOS from the pancreas tissue was quantified by western blot (Figure 4). Pretreatment of diosmetin significantly reduced the expression of iNOS in pancreas tissue.
Figure 4.

Antioxidant effect of diosmetin in caerulein-induced AP. Pancreatic tissue expression level of iNOS protein was detected by western blot (A, B). β-actin was used as the internal reference for total tissue proteins. Data are presented as mean ± SD. #P < 0.05 vs control group at the same time point, $P < 0.05 vs vehicle-treated group at the same time point. The results were similar in three additional experiments.
Diosmetin inhibits nuclear translocation of NF-κB
NF-κB activation plays a key role in the induction of several proinflammatory mediators [20]. The nuclear translocation of the NF-κB transcription factor is the sign of signal pathway activation. To determine the effect of diosmetin on NF-κB activity in pancreas, we examined the expression level of NF-κB p65 by western blot. As shown in Figure 5A, diosmetin reduced NF-κB p65 protein expression in mouse pancreas, especially obvious at the time point of 6 h after the first cerulein injection. Immunohistochemical analyses also confirmed that diosmetin inhibited NF-κB p65 nuclear translocation at the time point of 6 h after the first cerulein injection in mouse pancreas (Figure 5B).
Figure 5.
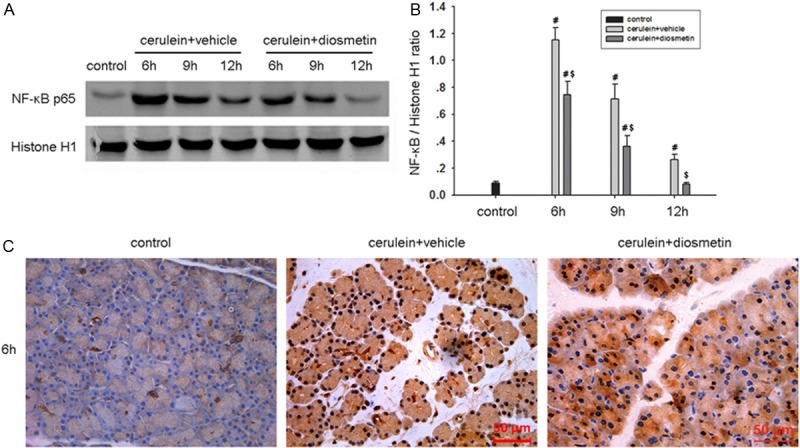
Diosmetin inhibits nuclear translocation of NF-κB. Pancreatic tissue expression level of NF-κB p65 protein in nucleus were detected by western blot (A, B). Histone H1 was used as the internal reference for nuclear proteins. Data are presented as mean ± SD. #P < 0.05 vs control group at the same time point, $P < 0.05 vs vehicle-treated group at the same time point. (C) Immunohistochemical analysis of NF-κB p65 in mouse pancreas at 6 h after induction of AP. Original magnifications: 400×.
Discussion
AP is a necroinflammatory pancreatic disease with high mortality and significant morbidity due to lack of specific therapy [21]. The development of AP is a multistep process, the exact mechanism of which remains controversial. It is widely held that intrapancreatic trypsinogen activation is the first step ultimately triggering the cascade of other digestive enzyme activation and is associated with TAP release already 15 min after pancreatitis induction in some models [22,23]. Injury to pancreatic acinar cells causes an increased oxidative stress resulting in the oxidation of lipids and proteins and disruption of the pancreatic membrane [24]. Production of nitric oxide by iNOS has been proposed as a pathogenic factor, proinflammatory role in AP, and iNOS-deficient mice exhibit resistance to the AP caused by cerulein in mice [25]. Although the onset of AP is triggered by early acinar events, its severity and systemic inflammation are determined by consecutive extra-acinar events through the recruitment and release of proinflammatory mediators, leading to a systemic inflammatory response. Recruitment of various inflammatory cells leads to further acinar cell injury and causes an elevation of various proinflammatory cytokines such as TNF-α, IL-1β and IL-6 [10]. Serum levels of proinflammatory cytokines have been found to correlate with the severity of AP. Additionally, it has been reported that the suppression of proinflammatory cytokines could ameliorate the severity of AP [26]. MPO produces hypochlorous acid (HOCl) from hydrogen peroxide (H2O2) and chloride (Cl-) during the neutrophil’s respiratory burst, so its activity has been used to determine quantitatively the extent of neutrophils infiltration. Various studies had clearly shown that diosmetin plays the role of anti-inflammatory activity and antioxidant activity in treating inflammatory diseases. The effects of diosmetin in these diseases could be explained by invoking different mechanisms, including the inhibition of cell cycle progression as a result of CYP1 enzyme mediated metabolism, prevention of LPS-induced up-regulation of NO production, reduction of proinflammatory cytokine secretion, suppression SCF-/UVB-induced melanogenesis by inhibit c-Kit signaling [27-30]. In the present study, we investigated the effect of diosmetin in a well-characterized model of AP induced by cerulein in mice which is very similar to human AP because of rapid inflammation development. Our observation demonstrated that diosmetin significantly alleviated the pancreatic damage in AP, as shown by histological features, TAP level and MPO activity, reduced the production of proinflammatory cytokines such as in serum and, inhibited the level of iNOS protein expression in pancreas.
The transcription factor NF-κB plays an important role in the pathogenesis of AP, and NF-κB is widely considered a key element in inflammatory responses based on its ability to regulate the expression of inflammatory mediators. Among the members of NF-κB family, NF-κB p65 is the crucial transcription factor of the classical pathway of NF-κB in AP [31]. Activation of NF-κB is an early event in pancreatitis paralleling and independent of trypsinogen activation [22]. The level of NF-κB activation correlates with the severity of cerulein-induced AP in mice [20]. In this study, we choose 6 h, 9 h and 12 h after the first cerulein injection as the time point to observe the activation of NF-κB respectively. By western blot, we observed that treatment of diosmetin could significantly attenuated the expression of NF-κB p65 in pancreatic nucleus during AP, which was especially obvious at the time point of 6 h. Furthermore, immunohistochemical analyses found that diosmetin inhibited NF-κB p65 nuclear translocation in mouse pancreas at 6 h after the first injection of cerulein.
Taken together, the study provides an evidence that diosmetin attenuates the severity of cerulein-induced AP by alleviating pancreatic tissue damage, reducing digestive enzyme production and proinflammatory cytokine secretion. Moreover, the inhibition of NF-κB activation is involved in the mechanisms of diosmetin effects on AP. It is possible that diosmetin treatment might provide a basis for new experimental study and clinical investigation of AP.
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (No. 81100317, No. 81370568 and No. 81270543), Foundation for Shanghai Science and Technology Committee (No. 12QA1402600, No. 114119a6800, No. 12411950600 and No. 11411950601) and outstanding Young Scholars Program of Shanghai Health System (No. XYQ2011004). No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ Failure and Infection of Pancreatic Necrosis as Determinants of Mortality in Patients With Acute Pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Awla D, Zetterqvist AV, Abdulla A, Camello C, Berglund LM, Spegel P, Pozo MJ, Camello PJ, Regner S, Gomez MF, Thorlacius H. NFATc3 Regulates Trypsinogen Activation, Neutrophil Recruitment, and Tissue Damage in Acute Pancreatitis in Mice. Gastroenterology. 2012;143:1352–1360. doi: 10.1053/j.gastro.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi H, Weidenbach H, Luhrs H, Lerch MM, Dickneite G, Adler G. Combined treatment with C1 esterase inhibitor and antithrombin III improves survival in severe acute experimental pancreatitis. Gut. 1997;40:531–535. doi: 10.1136/gut.40.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: Where are we now? Dig Dis. 2006;24:148–159. doi: 10.1159/000090318. [DOI] [PubMed] [Google Scholar]
- 5.Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439–1444. doi: 10.1136/gut.2006.115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakonczay Z, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-κB activation in the pathogenesis of acute pancreatitis. Gut. 2007;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Manabe T. A possible mechanism for gallstone pancreatitis: repeated short-term pancreaticobiliary duct obstruction with exocrine stimulation in rats. Proc Soc Exp Biol Med. 1993;202:246–252. doi: 10.3181/00379727-202-43534. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scand J Surg. 2005;94:97–102. doi: 10.1177/145749690509400203. [DOI] [PubMed] [Google Scholar]
- 9.Vonlaufen A, Apte MV, Imhof BA, Frossard JL. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213:239–248. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 10.Bakoyiannis A, Delis S, Dervenis C. Pathophysiology of acute and infected pancreatitis. Infect Disord Drug Targets. 2010;10:2–4. doi: 10.2174/187152610790410954. [DOI] [PubMed] [Google Scholar]
- 11.Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48:239–250. doi: 10.1111/j.1600-079X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Spanakis M, Kasmas S, Niopas I. Simultaneous determination of the flavonoid aglycones diosmetin and hesperetin in human plasma and urine by a validated GC/MS method: in vivo metabolic reduction of diosmetin to hesperetin. Biomed Chromatogr. 2009;23:124–131. doi: 10.1002/bmc.1092. [DOI] [PubMed] [Google Scholar]
- 13.Kanaze FI, Bounartzi MI, Niopas L. A validated HPLC determination of the flavone aglycone diosmetin in human plasma. Biomed Chromatogr. 2004;18:800–804. doi: 10.1002/bmc.391. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R, Chen Z, Jia G, Li J, Cai Y, Shao X. Protective effects of diosmetin extracted from Galium verum L. on the thymus of U14-bearing mice. Can J Physiol Pharmacol. 2011;89:665–673. doi: 10.1139/y11-058. [DOI] [PubMed] [Google Scholar]
- 15.Meng JC, Zhu QX, Tan RX. New antimicrobial mono- and sesquiterpenes from Soroseris hookeriana subsp. erysimoides. Planta Med. 2000;66:541–544. doi: 10.1055/s-2000-8607. [DOI] [PubMed] [Google Scholar]
- 16.Chandler D, Woldu A, Rahmadi A, Shanmugam K, Steiner N, Wright E, Benavente-Garcia O, Schulz O, Castillo J, Munch G. Effects of plant-derived polyphenols on TNF-alpha and nitric oxide production induced by advanced glycation endproducts. Mol Nutr Food Res. 2010;54(Suppl 2):S141–150. doi: 10.1002/mnfr.200900504. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez M, Avila JG, Nieto A, Cespedes CL. Anti-inflammatory activity of Penstemon gentianoides and Penstemon campanulatus. Pharm Biol. 2011;49:118–124. doi: 10.3109/13880209.2010.503708. [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Shen J, Cheng L, Guo C, Xu X, Wang F, Huang L, Yang L, He M, Xiang D, Zhu S, Wu M, Yu Y, Han W, Wang X. Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut. 2011;60:820–828. doi: 10.1136/gut.2010.215178. [DOI] [PubMed] [Google Scholar]
- 19.Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11. doi: 10.1016/s0014-2999(99)00421-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Liu Y, Daniluk J, Gaiser S, Chu J, Wang H, Li ZS, Logsdon CD, Ji B. Activation of nuclear factor-kappaB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144:202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 22.Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frossard JL. Trypsin activation peptide (TAP) in acute pancreatitis: from pathophysiology to clinical usefulness. JOP. 2001;2:69–77. [PubMed] [Google Scholar]
- 24.Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10–15. doi: 10.3109/00365529609104992. [DOI] [PubMed] [Google Scholar]
- 25.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Centorrino T, Ciccolo A, Van de Loo FA, Britti D, Caputi AP, Thiemermann C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416–422. doi: 10.1097/00024382-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:548–556. doi: 10.3748/wjg.v13.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21:1525–1528. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 28.Shanmugam K, Holmquist L, Steele M, Stuchbury G, Berbaum K, Schulz O, Benavente Garcia O, Castillo J, Burnell J, Garcia Rivas V, Dobson G, Munch G. Plant-derived polyphenols attenuate lipopolysaccharide-induced nitric oxide and tumour necrosis factor production in murine microglia and macrophages. Mol Nutr Food Res. 2008;52:427–438. doi: 10.1002/mnfr.200700180. [DOI] [PubMed] [Google Scholar]
- 29.Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chemistry. 2010;122:987–996. [Google Scholar]
- 30.Lee SJ, Jung TH, Kim H, Jeong D, Choi G, Park WK, Kong JY, Jin MH, Cho H. Inhibition of c-Kit signaling by diosmetin isolated from Chrysanthemum morifolium. Arch Pharm Res. 2014;37:175–185. doi: 10.1007/s12272-013-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treiber M, Neuhofer P, Anetsberger E, Einwachter H, Lesina M, Rickmann M, Liang S, Kehl T, Nakhai H, Schmid RM, Algul H. Myeloid, but not pancreatic, RelA/p65 is required for fibrosis in a mouse model of chronic pancreatitis. Gastroenterology. 2011;141:1473–1485. doi: 10.1053/j.gastro.2011.06.087. [DOI] [PubMed] [Google Scholar]




