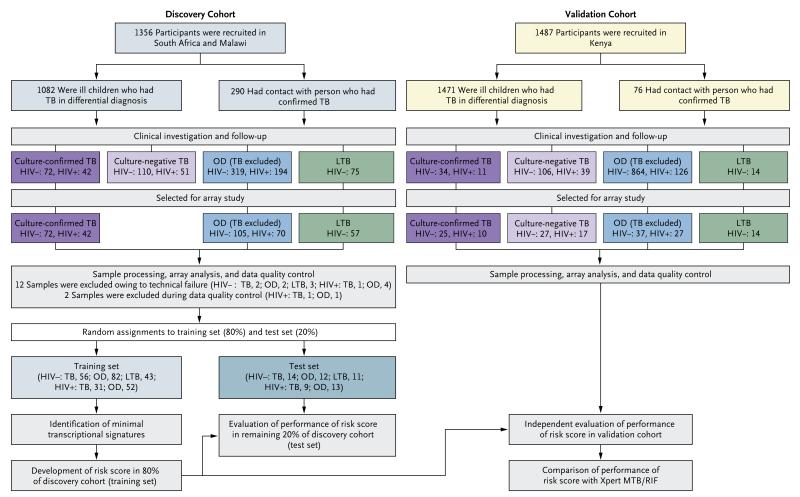
Figure 1. Overall Study Design and Numbers of Children Included in the Discovery and Validation Cohorts.
Patients in the discovery cohort were assigned to either the training set (80%) or the test set (20%); the signatures found in the discovery cohort were tested in the test set and then applied in the independent validation cohort. In the discovery cohort, 16 patients were excluded because of withdrawal of consent or inadequate sample collection; during clinical investigation and follow-up, samples were excluded because of inconclusive diagnoses; and during selection for array, samples were randomly selected. In the validation cohort, the patients with tuberculosis in differential diagnosis included 60 patients with features of tuberculosis on screening who had contact with a person with tuberculosis. HIV denotes human immunodeficiency virus, LTB latent tuberculosis (TB), and OD other diseases.