Figure 1.
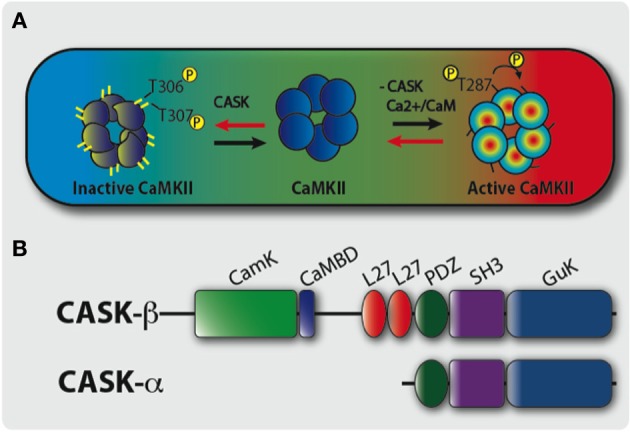
A model of CASK’s regulation of CaMKII autophosphorylation during memory formation. (A) The large colored rectangle represents a hypothetical neuron in the middle of which is a cartoon of a single layer of a CaMKII dodecamer holoenzyme. On the right, under conditions of increased synaptic activity (high [Ca2+], in red) Ca2+/CaM binds CaMKII via the CaM binding site that contains the inhibitory T306 T307 sites hence blocking them from autophosphorylation. This also promotes T287 autophosphorylation (pT287) and the switch to persistently high kinase activity even after Ca2+ levels fall. On the left, under conditions of low synaptic activity and low [Ca2+] (in blue), there is low probability of CaM binding to CaMKII allowing CASK to promote autophosphorylation of the inhibitory T306 T307 (pT306 pT307) sites. This renders the kinase inactive and even if there is a subsequent increase in Ca2+/CaM, CaM binding is blocked by pT306 pT307 in the CaM site. Eventually phosphatases will act to remove phosphorylation events and return endogenous CaMKII to its basal state. Therefore, in the absence of CASK there is a decrease in inhibitory pT306 pT307 and an increase in pT287 constitutively active CaMKII, conversely increased CASK promotes inhibitory pT306 pT307 decreasing pT287 and endogenous CaMKII activity. Therefore, neurons expressing transgenic CaMKII with inhibitory phosphorylation sites mutated to blocking residues (TT306/7AA) or with too little CASK due to mutation results in a form of CaMKII that is unable to switch off. This causes abnormally high CaMKII activity that subsequently interferes with the physiology of the neuron disrupting memory. (B) Predicted domain structure of CASK isoforms, the short isoform CASK-α contains PDZ, SH3, and GUK domains while the long isoform CASK-β contains additional CaMK-like (CamK), Calmodulin binding domain (CaMBD), and L27 domains at its N-terminus. The CASK-β null contains a N-terminal deletion removing CaMK, CaMBD, and L27 domains but leaves the downstream promoter and whole of CASK-α intact (Slawson et al., 2011).
