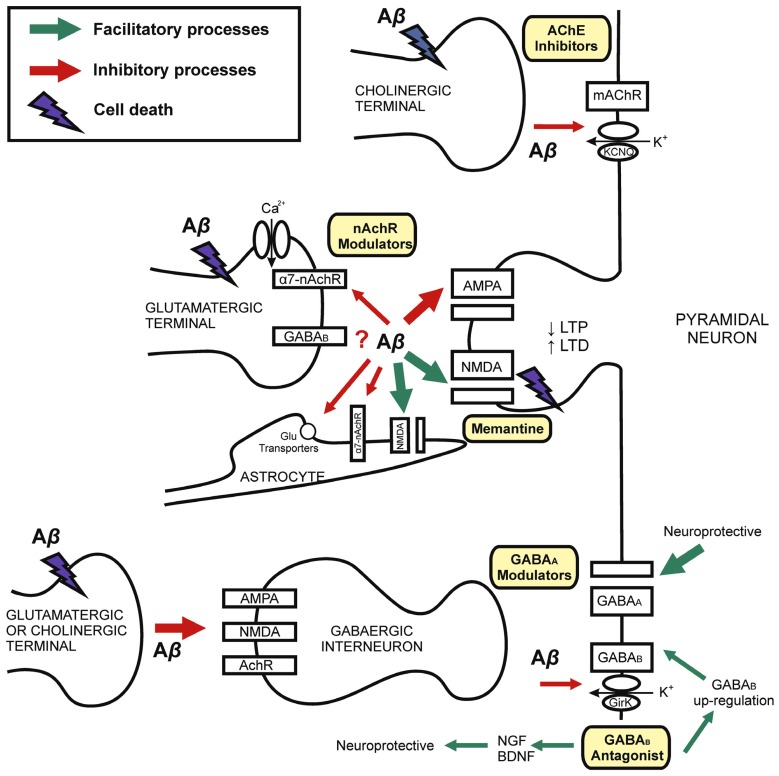
FIGURE 1.
Mechanisms of synaptic dysfunction in Alzheimer’s disease. Schematic drawing showing the role of Aβ in the physiopathology of AD and different targets for pharmacological modulation. AD is explained by Aβ-induced neurodegeneration, therefore with decline in synaptic terminals (left) on both principal cells and inhibitory interneurons. Chronic exposition to Aβ induces neuronal death. Synaptic loss is related with cognitive impairment in AD. Early memory decline is correlated with Aβ-synaptic dysfunction on excitatory and inhibitory receptors and neurotransmission. Current evidence for the treatment of AD involve both glutamatergic (NMDA-type glutamate receptors antagonist, i.e., memantine) and cholinergic (i.e., cholinesterase inhibitors, AChE inhibitors) transmission. The GABAergic system is a possible target for developing pharmacological interventions. GABAB receptors may be blocked by Aβ. However, GABAB antagonists induce GABAB receptors up-regulation and neurotrophic factors expression. GABAA modulation (i.e., by GABAA receptor agonist and selective inverse agonist) may have nootropic and neuroprotective effects. Although GABA interneurons are spared in several AD models, the imbalance between excitatory and inhibitory neurotransmission might underlie early AD cognitive dysfunction. Abbreviations: Aβ, Amyloid-β; AchR, acetylcholine receptor; AChE, acetyl-cholinesterase; α7-nAChR, α7-nicotinic acetylcholine receptor; BDNF, brain-derived neurotrophic factor; GirK, G protein-gated inwardly rectifying potassium channel; KCNQ, Kv7 family of voltage-gated potassium channels; LTP, long-term potentiation; LTD, long-term depression; NGF, nerve growth factor.