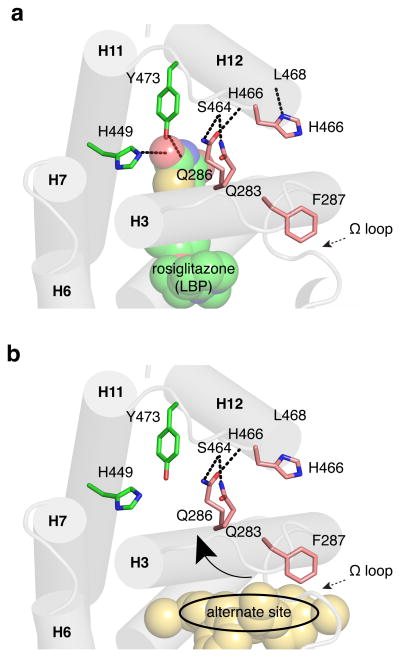
Figure 8. Possible structural mechanism for indirect stabilization of the PPARγ AF-2 surface via the alternate site.
(a) Ligands such as rosiglitazone that bind to the PPARγ canonical LBP form hydrogen bonds with residues in the helix 12 pocket (Y473 and H449) to directly stabilize helix 12 and the AF-2 surface. (b) Ligands that bind to the alternate site may indirectly stabilize the AF-2 surface by stabilizing helix 3, facilitating hydrogen bond formation between side chains of residues on helix 3 to residues in the helix 11-12 loop, particularly in the presence of a bound coregulator, which could affect helix 12/AF-2 stabilization.