Fig. 2.
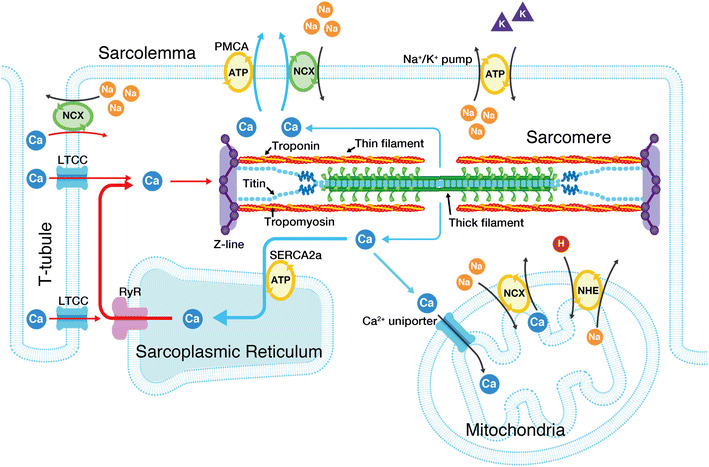
Schematic illustration indicating the structure of a cardiac sarcomere associated with the T-tubules. The influx of Ca2+ from the interstitial fluid during excitation causes the release of Ca2+ from the sarcoplasmic reticulum (SR). The released Ca2+ binds to troponin on the thin filaments and triggers sarcomeric contraction (systole) (see Fig. 3 for details). Relaxation (diastole) occurs as a result of uptake of Ca2+ by the SR Ca2+ pump, by extrusion of intracellular Ca2+ by Na+–Ca2+ exchangers, and partially by the sarcolemmal Ca2+ pump. Although the importance of mitochondria in pathophysiology has become increasingly evident, it remains unclear whether these organelles play a significant role in Ca2+ handling under physiological settings [e.g., 9]. The T-tubules and Z-lines run in parallel in cardiac muscle, causing Ca2+ sparks at/near the Z-lines [12, 13]. The thick and thin filaments, and titin are shown in this illustration (for simplicity, only two titin molecules per half thick filament are shown). Troponin and tropomyosin exist in the thin filaments, regulating actomyosin interaction in a [Ca2+]i-dependent manner. As described in detail in previous papers [e.g., 48–51], I-band titin is in a contracted state at the slack SL; straightening of the tandem Ig segment and then extension of the PEVK and N2B segments are thought to occur (resulting in passive force generation) in response to stretch. LTCC L-type Ca2+ channel, RyR ryanodine receptor, PMCA plasma membrane Ca2+ ATPase, NCX Na+–Ca2+ exchanger, NHE sodium–hydrogen exchanger
