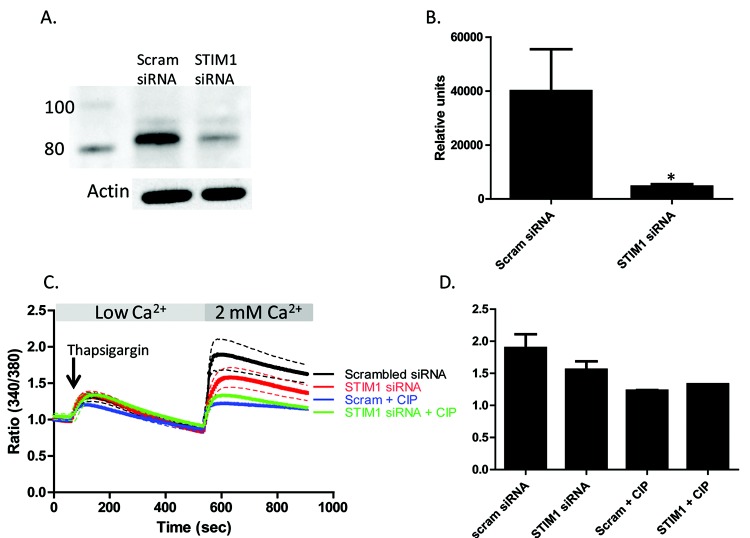
Figure 6.
Stromal interaction molecule 1 (STIM1) knockdown attenuates store-operated calcium (SOC) in pulmonary artery endothelial cells (PAECs). PAECs were transfected with 20 nM siRNA (small interfering RNA) to knockdown STIM1 using HiPerFect transfection reagent. Control cells received 20 nM scrambled (Scram) siRNA. A, Representative Western blots showing STIM1 levels in PAECs with and without siRNA treatment. Actin is shown as loading control. B, Densitometry analysis of siRNA-treated PAECs. An asterisk indicates significant differences from non-siRNA-treated control cells. C, Thapsigargin-induced SOC entry was decreased in PAECs treated with siRNA- and CIP-treated cells. PAECs received 20 nM of siRNA to knockdown STIM1 for >72 hours (or scrambled control). Cells were loaded with Fura 2/AM (fura 2-acetoxymethyl ester) and pretreated with 50 μM calcineurin inhibitory peptide (CIP) or vehicle alone for 1 hour, and changes in intracellular [Ca2+] were measured. SOC entry was initiated by thapsigargin treatment in low-Ca2+ buffer. Upon 2 mM Ca2+ add-back, cells treated with siRNA to STIM1 exhibited decreased SOC (red tracing) compared to controls (black tracing). CIP treatment had no effect on this decrease in SOC entry in STIM1 siRNA-treated cells (green tracing). Each tracing represents the average of at least 3 experiments (mean ± SEM) with 2 regions of interest of 10–20 cells for each. D, Graphical representation of calcium tracings in C at peak SOC entry (600 seconds).