Abstract Abstract
Formation of new blood vessels is essential for vascular repair and remodeling, and it is known that biomechanical properties of extracellular matrix play a major role in this process. Our earlier studies have also shown that exposing endothelial cells to oxidized modification of low-density lipoproteins (oxLDL) increases endothelial stiffness and facilitates their ability to form cellular networks, suggesting that it facilitates endothelial angiogenic potential. The goal of this study, therefore, was to test the interrelationship between matrix stiffness and oxLDL in the regulation of angiogenesis. Our results show that, as expected, an increase in matrix stiffness inhibited endothelial network formation and that exposure to oxLDL significantly facilitated this process. We also show, however, that oxLDL-induced facilitation of endothelial networks was observed only in stiff (3 mg/mL) but not in soft (1 mg/mL) collagen gels, resulting in blunting the effect of matrix stiffness. Also unexpectedly, we show that an increase in matrix stiffness results in a significant increase in the number of capillary lumens that are formed by single cells or pairs of cells, suggesting that while endothelial connectivity is impaired, formation of single-cell lumens is facilitated. oxLDL facilitates lumen formation, but this effect is also matrix dependent and is observed only in soft gels and not in stiff gels. Finally, an increase in both matrix stiffness and oxLDL exposure results in changes in capillary morphology, with the formation of larger capillary lumens. Overall, our study suggests that oxLDL plays an important role in formation of new capillaries and their morphology and that this effect is critically dependent on the extracellular environment’s compliance, thereby underlining the importance of the interdependence of these parameters.
Keywords: angiogenesis, matrix stiffness, oxidized low-density lipoprotein (oxLDL)
Introduction
The stiffness of the extracellular matrix has a major effect on angiogenesis, a process of formation of new capillaries,1-3 suggesting that tissue fibrosis plays an important role in the abnormalities of capillary formation. A clinical example is progressive systemic sclerosis (PSS), or scleroderma, a disease that is characterized by loss and distortion of capillaries and other small blood vessels,4,5 as well as extensive collagen deposition and profound stiffness of several organ systems.6 Furthermore, a growing number of studies have shown that multiple rheumatologic diseases, including PSS, are associated with increased susceptibility of low-density lipoprotein (LDL) to oxidation and increased levels of oxidative modifications of low-density lipoprotein (oxLDL)7,8 (reviewed by Levitan et al.9), a proinflammatory factor that is believed to cause endothelial dysfunction,9-12 but its role in dysregulation of angiogenesis in the presence of tissue fibrosis is unknown. Our studies have shown that oxLDL facilitates formation of endothelial networks in an in vitro model of angiogenesis, suggesting that it enhances endothelial angiogenic potential.13,14 These observations were further supported by other studies showing that oxLDL may enhance tube formation, another assay for neovascularization.15 In this study, we investigate the relationship between tissue stiffness and oxLDL in the ability of endothelial cells (ECs) to form endothelial networks and capillary lumens.
The rationale for a possible relationship between matrix stiffness and oxLDL exposure is based on recent studies demonstrating not only that the stiffness of the extracellular matrix is important for the formation of the capillaries but also that the biomechanical properties of ECs themselves appear to affect capillary formation.13,16,17 More specifically, while increase in tissue stiffness was shown to have an inhibitory effect on the ability of ECs to form networks in 3-dimensional (3D) collagen gels17,18 and grow vascular sprouts into the matrix milieu,16 an increase in stiffness of ECs themselves appears to have the opposite effect, increasing the ability of ECs to form networks.17 Consistent with this idea, we have shown previously that oxLDL-induced facilitation of endothelial network formation is associated with the increase in endothelial stiffness.13,14 It is also important to note, however, that the impact of tissue stiffness on capillary formation may be complex, as suggested by a biphasic relationship observed in Matrigel plug assay by Mammoto et al.19
We suggest, therefore, that exposure to oxLDL may alter the sensitivity of the cells to matrix stiffness. In this study, we test this hypothesis by investigating how exposure to oxLDL affects endothelial network and lumen formation, as well as capillary morphology in matrices of different amounts of stiffness. Our observations show that the impact of oxLDL on endothelial network formation and lumen generation depends on the underlying matrix stiffness and that exposure to oxLDL blunts the effects of matrix stiffness on capillary formation. These studies indicate that there is a complex relationship between oxLDL and matrix stiffness in the regulation of angiogenesis.
Material and methods
Cell culture and reagents
Bovine aortic endothelial cells (BAECs; Cambrex, East Rutherford, NJ) were grown between passages 5 and 20 in Delbeccio’s modified eagle medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 10 μg/mL penicillin, streptomycin, and kanamycin sulfate (Invitrogen). Cell cultures were maintained in a humidified incubator at 37°C with 5% CO2. Cells were split every 3–4 days, oxLDL (Biomedical Technologies, Stoughton, MA) was dissolved in DMEM to a final concentration of 10 μg/mL, and thiobarbituric acid–reactive (TBAR) substances were assayed as a measure of oxidative lipid modification (18–26 nmol malondialdehyde equivalents per milligram of LDL protein).
oxLDL treatment
BAECs were first exposed to 10 μg/mL oxLDL (10–15 nmol/mg protein TBAR) for 1 hour as described in our previous studies13,14 and then imbedded into gels of increasing stiffness.
Gel preparation and network visualization
Collagen gels were prepared according to manufacturer’s instructions to a final collagen concentration of 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL (Becton Dickinson, Franklin Lanes, NJ). BAECs were seeded into gel mixtures at 6–8 × 105 cells/mL, and gels were allowed to polymerize for 20 minutes at 37°C in 48-well plates. Thereafter, the gels were mechanically loosened from the sides of the wells and growth media supplemented with vascular endothelial growth factor obtained from Pepro Tech (Rocky Hill, NJ), basic fibroblast growth factor, and phorbol myristate acetate at concentrations of 50 μg/mL each, obtained from Sigma. Gels were cultured for 2 days, and cells were visualized by fixing in 4% paraformaldehyde at 4°C overnight and stained with 0.1% toluidine blue (for 5 minutes). Images were obtained at 10× magnification (Nikon, Eclipse TE200-U microscopic image capturing system) to observe the EC network formation, elongation, and interconnectivity. The images were analyzed using the Scion Image analysis system. Briefly, 5 images were taken per gel from 4 gels per each experimental condition, made binary using a threshold value, and skeletonized using built-in Scion image functions as described previously.17
Lumen visualization and quantification
ECs embedded in 3D collagen gels form lumens that are clear of collagen, and these lumens are clearly visible by histological examination of the gel sections.17,20 To evaluate lumen formation, fixed gels were processed for histology and stained with hematoxylin and eosin to visualize collagen and cells in histological sections. Lumens were defined as matrix-free regions surrounded by contiguous cell regions. Four gels were analyzed for each experimental condition in each of the 3 experiments; 2 histological sections for each gel were analyzed. Lumen numbers were obtained by manually counting each histological section under a Zeiss microscope. This was verified by 2 independent researchers who were masked to the conditions of the experiment.
Statistical analysis
Statistical significance was evaluated using a standard t test assuming 2-tailed distributions with unequal variance.
Results
oxLDL attenuates the impact of matrix stiffness on endothelial cell network formation
Formation of endothelial networks in 2- and 3D cultures is one of the most frequently used methods to assess angiogenesis in vitro. To determine the effect of matrix stiffness on EC network formation, BAECs were seeded into collagen gels with increasing concentration of collagen (1–3 mg/mL) corresponding to an increase in matrix stiffness from about 100 to 600 Pa, as described earlier.21 The same number of cells (100,000/gel) were seeded into each gel at the beginning of the experiment. As expected, the degree of cell connectivity and elongation was significantly reduced as the gel stiffness increased. The softer gels (1–1.5 mg/mL collagen) had extensive EC connections, giving the appearance of a web with lacelike strands going in every direction and with multiple connections. Moreover, in soft gels, most cells are elongated and seem to be overlapping and confluent. In contrast, in stiff gels (2–3 mg/mL collagen), many cells look round, and there is clearly less connections between cells. The difference is apparent from the images of the EC networks within the gels (Fig. 1A).
Figure 1.
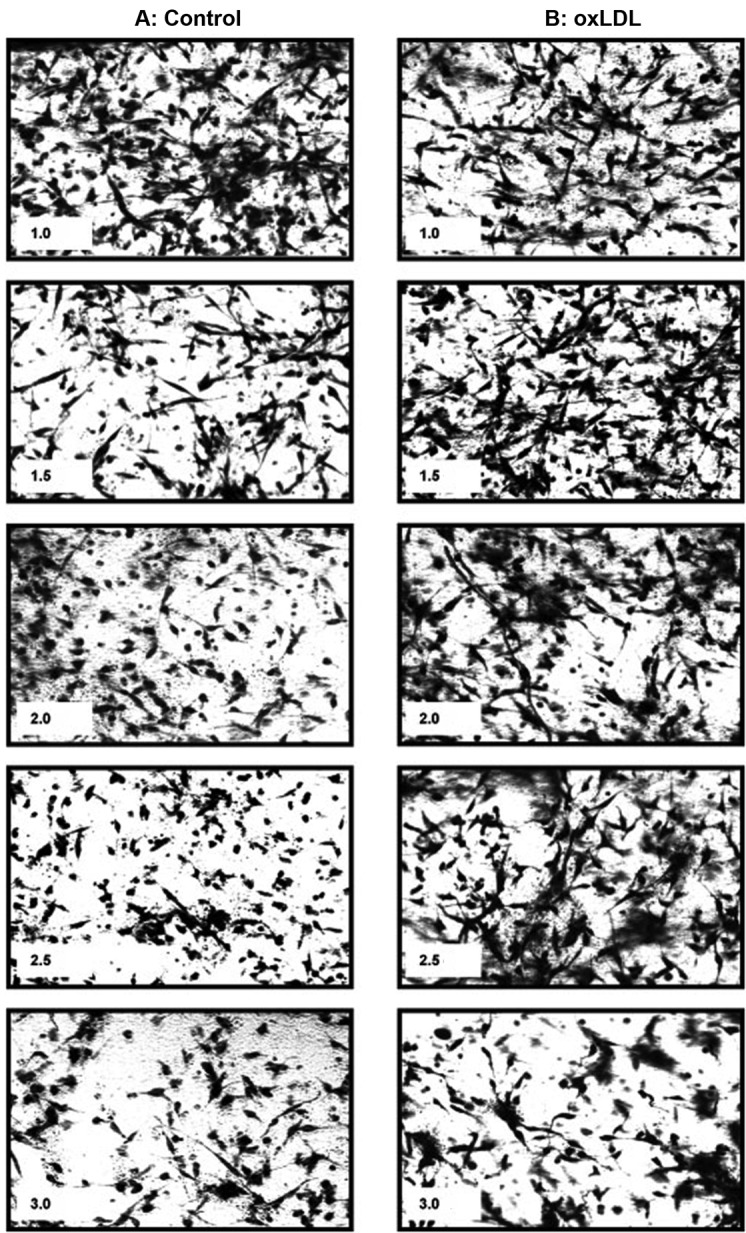
Representative images of endothelial networks in matrices of different stiffness in control cells (A) and in cells preexposed to 10 μg/mL oxidized low-density lipoprotein (oxLDL; B), demonstrating a negative impact of collagen concentration on the ability of cells to generate networks that is partially alleviated by the preexposure to oxLDL. Cells are embedded in 3-dimensional collagen gels with increasing collagen concentration (1.0–3.0 mg/mL), as specified in each image. The collagen concentration is specified in a text box included in each image (1.0, 1.5, 2.0, 2.5, 3.0).
To investigate the interaction between oxLDL and matrix stiffness on EC network formation, BAECs were first exposed to 10 μg/mL oxLDL (10–15 nmol/mg protein TBAR) for 1 hour as described in our previous studies13,14 and then imbedded into gels of increasing stiffness, as described above. Consistent with our earlier studies,13,14 exposure to oxLDL facilitated EC network formation, but this effect was not homogenous across the different levels of gel stiffness (Fig. 1B). Specifically, oxLDL promoted network formation in stiffer gels (2–3 mg/mL collagen), but little or no effect was observed in softer gels. Overall, the inhibitory effect of matrix stiffness on EC network formation appears to be blunted by preexposing the cells to oxLDL.
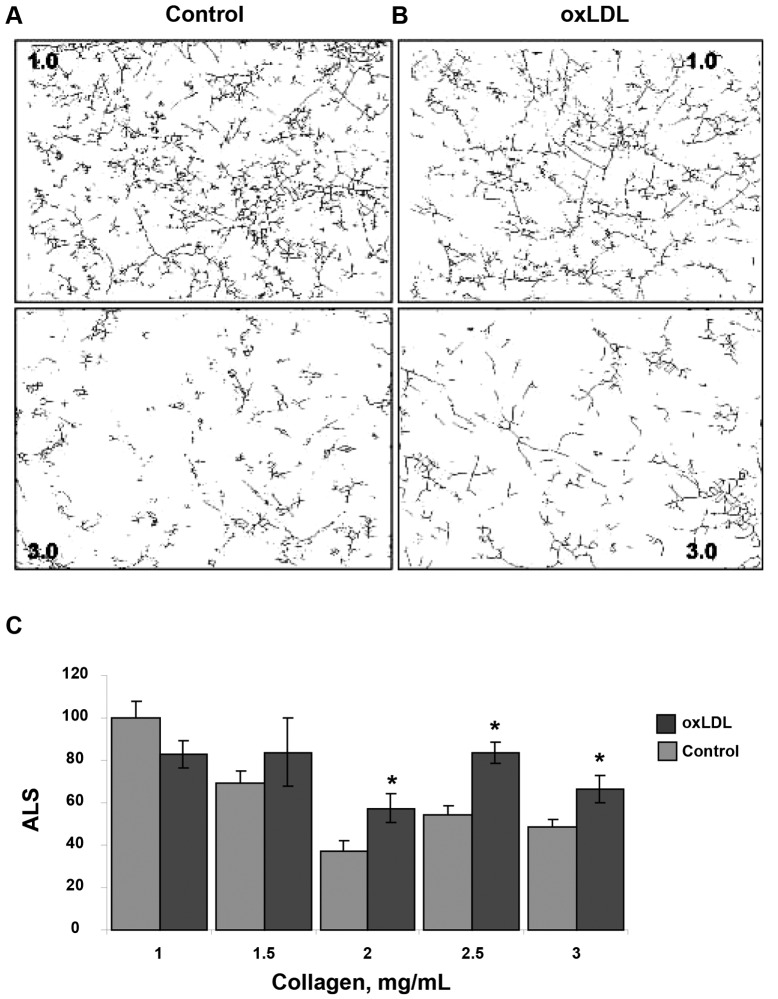
To quantify these effects, the images were skeletonized to evaluate the morphogenesis and interconnectivity of the ECs in the gel, as described earlier.13,17 The more elongated the cells are and the more connections they form, the longer uninterrupted lines are in the skeletonized images. Figure 2A, 2B shows representative skeletonized images of cells imbedded into 1.0- and 3.0-mg/mL collagen gels (upper and lower panels, respectively) in control cells (A) and oxLDL-treated cells (B). The average length of the skeletonized structure (ALS), a measure of the length of uninterrupted line, is an index of network formation. As expected,17 an increase in gel stiffness resulting from increasing the collagen concentration from 1 to 3 mg/mL decreased the ALS index from 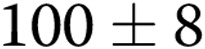 pixels in soft gels (1 mg/mL) to
pixels in soft gels (1 mg/mL) to 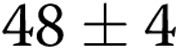 pixels in stiff gels (3 mg/mL; 50% decrease;
pixels in stiff gels (3 mg/mL; 50% decrease; 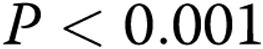 ), thus indicating a significant reduction in the ability of BAECs to form endothelial networks. However, preexposure to oxLDL resulted in significant increase in the ALS index in gels with the collagen concentrations 2–3 mg/mL when compared to controls (cells that were not exposed to oxLDL;
), thus indicating a significant reduction in the ability of BAECs to form endothelial networks. However, preexposure to oxLDL resulted in significant increase in the ALS index in gels with the collagen concentrations 2–3 mg/mL when compared to controls (cells that were not exposed to oxLDL; 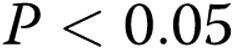 ; Fig. 2C ).
; Fig. 2C ).
Figure 2.
Effect of oxidized low-density lipoprotein (oxLDL) on endothelial network formation in gels of different collagen concentrations. A, B, Representative skeletonized images of endothelial network shown in 1.0- and 3.0-mg/mL collagen gels for control cells (A) and for cell preexposed to 10 μg/mL oxLDL (B), demonstrating that the network is significantly suppressed in 3.0-mg/mL collagen gels as compared to 1.0-mg/mL gels. The upper panels show the images of the network in soft gels (1.0 mg/mL), and the lower panels show the images in stiff gels (3.0 mg/mL). C, Average length of skeletonized image (ALS), an index of cell elongation and network connectivity, as a function of collagen gel concentration for control and oxLDL-treated cells. All values are normalized to the ALS index of control cells seeded in gels of the lowest stiffness (1 mg/mL collagen) in the same experiment. The data show mean ± standard error of the mean; an asterisk shows significance between control and oxLDL-treated cells (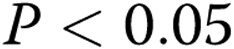 ).
).
Analysis of lumen formation as a function of matrix stiffness and oxLDL exposure
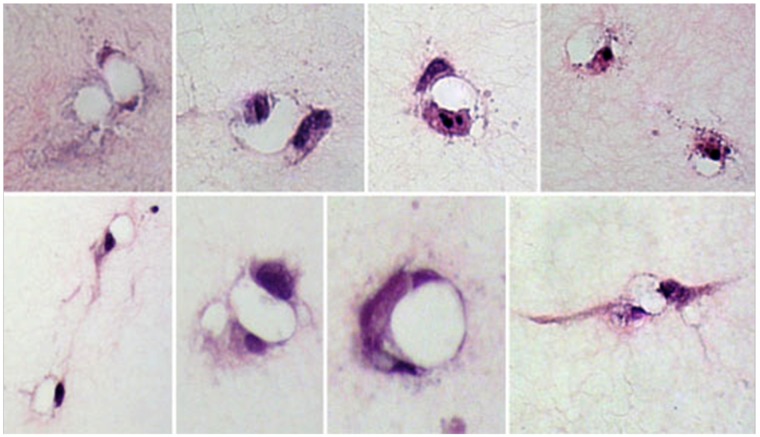
Multiple lumens were formed by the ECs in every gel. Most lumens developed from a single cell or a pair of adjacent cells, and their morphology resembled the neocapillary structure seen in vivo. The lumens varied in size and shape, with the lumen inner areas varying between 50 and 200 μm2. The lumens were mostly circular or ovoid; some were simple, and others were associated with cytoplasmic extensions. However, they all met the definition criteria specified in “Material and methods” by exhibiting lack of collagen. Representative images of different lumen shapes and configurations are shown in Figure 3.
Figure 3.
Representative images of endothelial cell lumens (matrix-free regions surrounded by cellular regions) formed in the collagen gels demonstrates the various shapes and sizes of lumens formed, with cellular regions being made up of 1 or more endothelial cells and their extensions. The images were taken at 200×.
Unexpectedly, in contrast to the inhibitory effect of increased gel stiffness on endothelial network formation, the ability of the cells to generate individual lumens was facilitated (Fig. 4A). In the control group, the number of lumens increased from 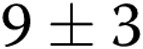 to
to 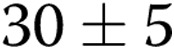 per slide (
per slide (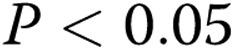 ) as the gel stiffness increased from 1 to 2 mg/mL collagen. Further increase in gel stiffness did not have a significant effect. In ECs treated with oxLDL, we observed the same trend, albeit to a lesser degree, with the number of lumens increasing from
) as the gel stiffness increased from 1 to 2 mg/mL collagen. Further increase in gel stiffness did not have a significant effect. In ECs treated with oxLDL, we observed the same trend, albeit to a lesser degree, with the number of lumens increasing from 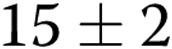 to
to 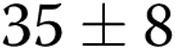 per slide (
per slide (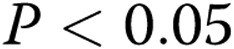 ) as the gel stiffness increased from 1 to 2 mg/mL collagen. An increase in gel stiffness above 2 mg/mL collagen did not have a significant effect on the number of lumens generated by oxLDL-treated cells, similar to the effect found for control cells. It is also important to note that in soft gels (1–1.5 mg/mL collagen), the number of lumens formed by oxLDL-treated cells was greater than that formed by control cells and increased in parallel to the control group. No significant difference was observed, however, in the number of lumens formed by the control and oxLDL-treated cells in stiff gels (2–3 mg/mL collagen; Fig. 4B).
) as the gel stiffness increased from 1 to 2 mg/mL collagen. An increase in gel stiffness above 2 mg/mL collagen did not have a significant effect on the number of lumens generated by oxLDL-treated cells, similar to the effect found for control cells. It is also important to note that in soft gels (1–1.5 mg/mL collagen), the number of lumens formed by oxLDL-treated cells was greater than that formed by control cells and increased in parallel to the control group. No significant difference was observed, however, in the number of lumens formed by the control and oxLDL-treated cells in stiff gels (2–3 mg/mL collagen; Fig. 4B).
Figure 4.
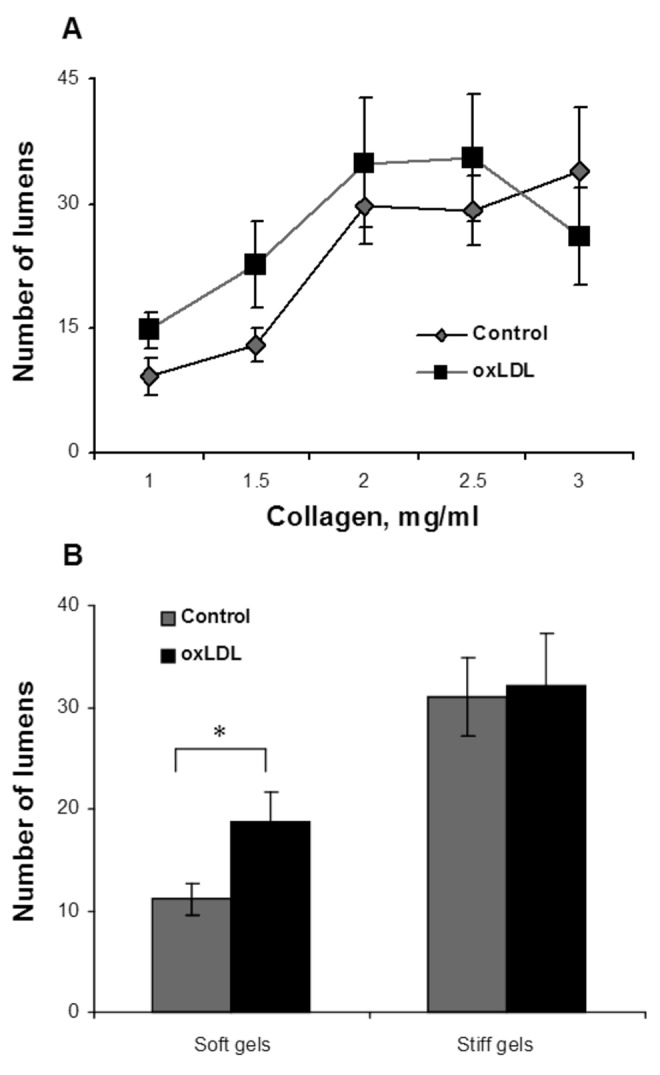
Analysis of lumen quantity as a function of gel collagen concentration and oxidized low-density lipoprotein (oxLDL). A, Average number of lumens formed per gel by oxLDL-treated cells (black squares) and control cells (gray diamonds) as a function of collagen concentration, demonstrating an increase in the number of lumens with the increase in gel collagen concentration. B, Pooled data for lumen number in soft (1.0- and 1.5-mg/mL collagen concentration) and stiff (2.0–3.0-mg/mL collagen concentration) gels, demonstrating a significant increase in the number of lumens in stiff gels versus soft gels, as well as a significant increase in oxLDL-treated cells observed in soft gels but not stiff gels. The data show mean ± standard error of the mean (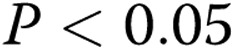 ).
).
Analysis of lumen morphology as a function of matrix stiffness and oxLDL exposure
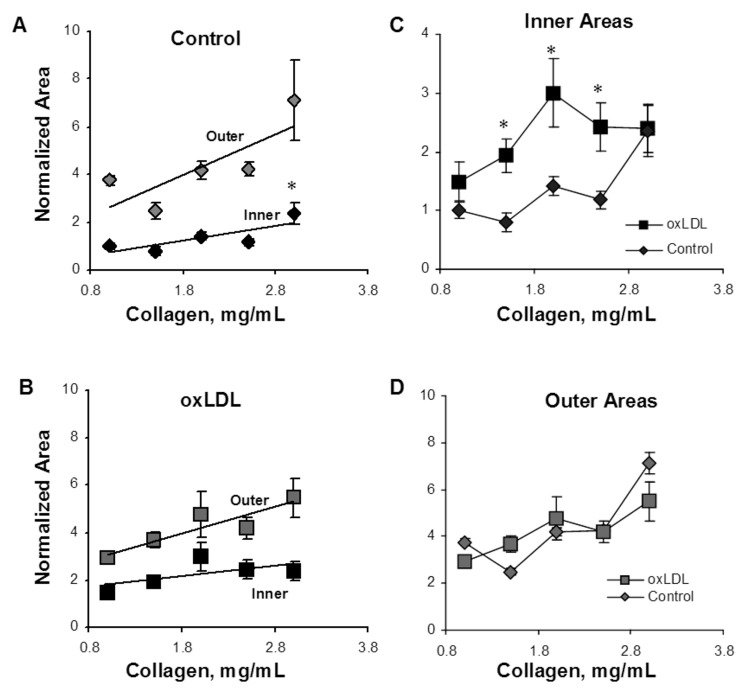
Capillary morphology is also affected significantly by both the stiffness of the matrix and oxLDL. Specifically, we measured 2 morphological features of the lumens that formed within the gels: the inner area of lumen was defined as the collagen-free and cell-free area within the cellular ring and represents the lumen of the capillary; the outer area was defined as the area of the lumen and the ECs that form it. The difference between the outer and inner areas represents the thickness of the capillary wall. Consistent with the earlier studies,17 our data show that an increase in matrix stiffness results in a 2.4-fold increase in the lumen areas of the capillaries (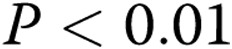 ; Fig. 5A). Furthermore, we also show here that addition of oxLDL resulted in a further significant increase in the areas of the lumens,with the effect being significant in the midrange of gel stiffness (1.5–2.5 mg/mL), thereby augmenting the effect of matrix stiffness (Fig. 5C;
; Fig. 5A). Furthermore, we also show here that addition of oxLDL resulted in a further significant increase in the areas of the lumens,with the effect being significant in the midrange of gel stiffness (1.5–2.5 mg/mL), thereby augmenting the effect of matrix stiffness (Fig. 5C; 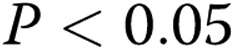 ).
).
Figure 5.
Analysis of lumen morphology as a function of gel collagen concentration and oxidized low-density lipoprotein (oxLDL). A, Inner area, which represents the area of the capillary lumen (black diamonds), and the outer area (gray diamonds), which represents the lumen and endothelial cells, or total area of the capillary. The inner area increases significantly with increasing gel collagen content (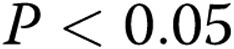 ), and the outer area shows a similar trend (
), and the outer area shows a similar trend (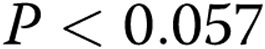 ). The difference between the inner and outer areas represents cell wall thickness, or endothelial cell area, which shows a trend to increase with the gel collagen concentration. B, Inner (black squares) and outer (gray squares) areas in cells treated with oxLDL demonstrated the same trend but a reduced difference between inner and outer areas corresponding to a decrease in cell wall thickness. C, Overlapping the areas of the lumens of control (black diamonds) and oxLDL-treated (black squares) cells demonstrates that oxLDL-treated cells form larger lumens than do control cells in most stiffnesses (
). The difference between the inner and outer areas represents cell wall thickness, or endothelial cell area, which shows a trend to increase with the gel collagen concentration. B, Inner (black squares) and outer (gray squares) areas in cells treated with oxLDL demonstrated the same trend but a reduced difference between inner and outer areas corresponding to a decrease in cell wall thickness. C, Overlapping the areas of the lumens of control (black diamonds) and oxLDL-treated (black squares) cells demonstrates that oxLDL-treated cells form larger lumens than do control cells in most stiffnesses (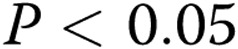 ) except for the very soft and the very stiff gels. D, Overlapping the outer areas of control (gray diamonds) and oxLDL-treated (gray squares) cells demonstrates that oxLDL has no effect on this parameter.
) except for the very soft and the very stiff gels. D, Overlapping the outer areas of control (gray diamonds) and oxLDL-treated (gray squares) cells demonstrates that oxLDL has no effect on this parameter.
The outer areas of the capillaries show the same trend as the lumens, increasing with an increase in matrix stiffness (Fig. 5D). Furthermore, the difference between the lumens and the outer area, a measure of capillary wall thickness, also increases as the gels become stiffer (Fig. 5A). However, unlike the lumens, the outer areas did not change when ECs were exposed to oxLDL (Fig. 5D), indicating that the EC layer and, thus, capillaries were thinner, as is apparent from comparing Figure 5A and Figure 5B.
Discussion
It is well accepted that biomechanical properties of the extracellular environment play a major role in the regulation of angiogenesis.1,2,16-18,22 It is less clear, however, how changes in matrix biomechanics affect the sensitivity of ECs to soluble pro- or antiangiogenic factors. In this study, we show that there is a complex and interdependent relationship between oxLDL, a major proinflammatory factor that has been shown to be both anti- and proangiogenic in previous studies,13,15,23 and matrix stiffness in regulating the angiogenic potential of ECs. More specifically, our observations show that exposure to oxLDL tends to blunt the effect of matrix stiffness on endothelial morphogenesis and the ability of the ECs to form networks and that, conversely, an increase in matrix stiffness blunts the effect of oxLDL on the formation of capillary lumens.
Angiogenesis depends on the ability of the cells to proliferate, migrate, interconnect, and form capillary lumens. Angiogenic potential of ECs, therefore, is typically assayed in vitro by quantifying endothelial networks that form either on 2D or within 3D gels.20,24 The advantage of the 3D model is that it allows generation of capillary lumens that resemble capillaries that form in vivo. This method, therefore, has been widely used to elucidate the signaling pathways that control EC morphogenesis and lumen formation.20,25 Similar to previous studies,17 we show here that an increase in gel stiffness results in a significant decrease in endothelial network formation. However, preexposing the cells to oxLDL attenuates the deleterious effect of increased matrix stiffness on network formation, as is apparent from the increased network formation in the stiffer gels that were exposed to oxLDL compared to controls. It is important to note that a lack of oxLDL effect in soft gels could be due to efficient network formation in soft pliable gels, so that the ability of the cells to generate networks reaches its limitation even before the addition of oxLDL. The range of gel stiffness explored in this study is 100–600 Pa,21 which corresponds to the stiffness of the lung tissue.26 In terms of the mechanism, one possible explanation for this blunted response is that oxLDL increases endothelial stiffness, and the ability of the cells to generate force on cell-substrate interface,13,14 which as was proposed earlier,13,17 may counteract the deleterious effect of matrix stiffness on EC network formation.
The impact of gel stiffness on lumen formation, however, is actually the opposite of that on the EC network, as we see a significant increase in the number of lumens in stiffer gels compared to soft ones. This is in contrast to previous studies, showing that the number of cell-derived blood vessels that form in collagen plugs embedded into immunodeficient mice decreases with an increase in collagen concentration and gel stiffness.27 It is important to note, however, that while in terms of collagen concentration of the gels the two studies are similar, the morphology of the blood vessels is completely different. As described above, the lumens that were observed in our study were relatively homogenous in size, being formed from either single cells or pairs of cells with the size distribution between 50 and 200 μm2, as was described in earlier studies.3,25,28 It was also shown earlier that these lumens are formed from intracellular vacuoles that subsequently coalesce to form intracellular lumens, which then merge further to form multicellular capillary tubes.3,25,28 In contrast, blood vessels that were observed by Critser et al.27 in collagen plugs in vivo were highly variable, ranging from small vessels to large multicellular structures of up to 4,000 μm2. We suggest, therefore, that an increase in collagen has a differential effect on the formation of single-cell capillaries observed in our study versus multicellular structures observed previously in vivo.27 Furthermore, the opposite effects of matrix stiffness on endothelial connectivity and lumen formation observed in our study suggest that endothelial extensions, which represent early tubule formation, are not tightly coupled with the number of capillary lumens and that the two processes are regulated independently. Indeed, earlier studies suggested that different Rho-GTPases are involved in the control of endothelial elongation and branching, regulated by RhoA, versus lumen formation, regulated by Rac 1 and Cdc42.25
Another finding of this study is not only that the exposure to oxLDL affects the sensitivity of the cells to changes in matrix stiffness but also that the response of ECs to oxLDL is strongly affected by changes in matrix stiffness. Indeed, oxLDL had no effect on EC network formation in soft gels but had a significant facilitatory effect in stiffer gels. Conversely, oxLDL facilitated lumen formation in soft gels but had no effect in stiff gels. Finally, exposure to oxLDL results in the formation of larger lumens, augmenting the effect of an increase in matrix stiffness alone. This effect, however, is also matrix dependent. These observations highlight the possibility that sensitivity of ECs to soluble pro- or antiangiogenic factors might depend on the biomechanical properties of the matrix. Overall, a combination of matrix stiffness and oxLDL results in formation of an increased number lumens that are abnormally large and with less connectivity. These findings might be of major physiological significance for understanding the vascular repair process under different pathological conditions.
Physiological/pathological significance. A clinical example of the interplay between matrix stiffness and angiogenesis can be found in scleroderma, a disease of uncontrolled fibrosis and blood vessel restriction and chaotic angiogenesis in target organs.29 The nailfold vasculature of this disease shows capillary dropout and large, irregularly shaped capillaries.30,31 Other target organs show similar changes.32 Indeed, a characteristic feature of scleroderma vascular lung disease is the “onion-skin” restriction around arterioles from intimal fibrosis. Another hallmark of scleroderma is cutaneous telangiectasias. Telangiectasias, which are essentially giant and aberrant capillaries, are present on the pleura and other organs and may well be another example of “capillary dysplasia” associated with increased matrix stiffness. Our observations show that tissue stiffness and proinflammatory oxidized lipids affect ECs in an interconnected manner. Yet, the angiogenic activity associated with increased capillary lumen numbers appears to be unconnected to that associated with capillary lumen size, a relationship we suggest is related to the matrix and EC state.
Source of Support: This work was supported by National Institutes of Health grants HL073965 and HL083298 to IL.
Conflict of Interest: None declared.
References
- 1.Ingber DE. Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin Cancer Biol 1992;3:57–63. [PubMed]
- 2.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol 1989;109:317–330. [DOI] [PMC free article] [PubMed]
- 3.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol 2011;3:a005090. [DOI] [PMC free article] [PubMed]
- 4.Beyer C, Schett G, Gay S, Distler O, Distler J. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther 2009;11:220. [DOI] [PMC free article] [PubMed]
- 5.Wigley FM. Vascular disease in scleroderma. Clin Rev Allergy Immunol 2009;36:150–175. [DOI] [PubMed]
- 6.Klippel JH, Stone JH, Crofford LJ, White PH, eds. Primer on the rheumatic diseases. Atlanta: Arthritis Foundation; 2001.
- 7.Bruckdorfer KR, Hillary JB, Bunce T, Vancheeswaran R, Black CM. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis. Arthritis Rheum 1995;38:1060–1067. [DOI] [PubMed]
- 8.Herrick AL, Illingworth KJ, Hollis S, Gomez-Zumaquero JM, Tinahones FJ. Antibodies against oxidized low-density lipoproteins in systemic sclerosis. Rheumatology 2001;40: 401–405. [DOI] [PubMed]
- 9.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition and pathophysiology. Antiox Redox Signal 2010;13:39–75. [DOI] [PMC free article] [PubMed]
- 10.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 2006;185:219–226. [DOI] [PubMed]
- 11.Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther 2011;25:419–429. [DOI] [PubMed]
- 12.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 2007;7:257–264. [DOI] [PubMed]
- 13.Byfield FJ, Tikku S, Rothblat GH, Gooch KJ, Levitan I. OxLDL increases endothelial stiffness, force generation and network formation. J Lipid Res 2006;47:715–723. [DOI] [PubMed]
- 14.Shentu TP, Titushkin I, Singh DK, Gooch KJ, Subbaiah PV, Cho M, Levitan I. oxLDL-induced decrease in lipid order of membrane domains is inversely correlated with endothelial stiffness and network formation. Am J Physiol Cell Physiol 2010;299:C218–C229. [DOI] [PMC free article] [PubMed]
- 15.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1 dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol 2007;27:2435–2442. [DOI] [PubMed]
- 16.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol 2009;297:C179–C187. [DOI] [PubMed]
- 17.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res 2004;297:574–584. [DOI] [PubMed]
- 18.Kanzawa S, Endo H, Shioya N. Improved in vitro angiogenesis model by collagen density reduction and the use of type III collagen. Ann Plast Surg 1993;30:244–251. [DOI] [PubMed]
- 19.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009;457:1103. [DOI] [PMC free article] [PubMed]
- 20.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol 2008;443:83–101. [DOI] [PubMed]
- 21.Byfield FJ, Reen RK, Shentu T-P, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 2009;42:1114. [DOI] [PMC free article] [PubMed]
- 22.Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol 2007;293:H3650–H3658. [DOI] [PMC free article] [PubMed]
- 23.Wang DY, Yang VC, Chen JK. Oxidized LDL inhibits vascular endothelial cell morphogenesis in culture. In Vitro Cell Dev Biol Anim 1997;33:248–255. [DOI] [PubMed]
- 24.McGonigle S, Shifrin V. In vitro assay of angiogenesis: inhibition of capillary tube formation. Curr Protocol Pharmacol 2008;43:12.12.11–12.12.17. [DOI] [PubMed]
- 25.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec 2002;268:252. [DOI] [PubMed]
- 26.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010;190:693–706. [DOI] [PMC free article] [PubMed]
- 27.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res 2010;80:23. [DOI] [PMC free article] [PubMed]
- 28.Davis GE, Bayless KJ. An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation 2003;10:27. [DOI] [PubMed]
- 29.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009;360:1989–2003. [DOI] [PubMed]
- 30.Cutolo M, Pizzorni C, Sulli A. Nailfold video-capillaroscopy in systemic sclerosis. Z Rheumatol 2004;63:457–462. [DOI] [PubMed]
- 31.Cutolo M, Sulli A, Pizzorni C, Sulli A. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000;27:155–160. [PubMed]
- 32.Mulligan-Kehoe MJ, Simons M. Vascular disease in scleroderma: angiogenesis and vascular repair. Rheum Dis Clin North Am 2008;34:73–79. [DOI] [PubMed]