Figure 6.
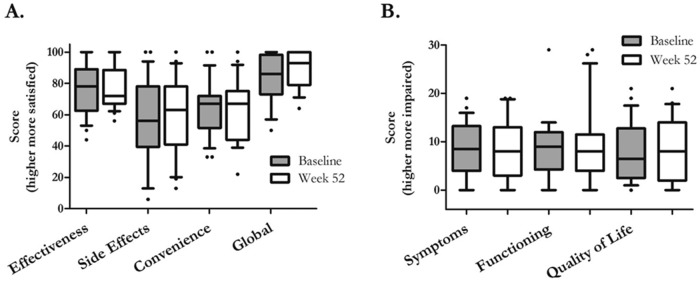
Treatment satisfaction and quality of life. Treatment satisfaction was measured at the beginning and end of the 52-week study using the Treatment Satisfaction Questionnaire for Medicine (TSQM)29 and quality of life by the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR).30 In A, patients scored subcutaneous treprostinil well for efficacy but less so for side effects and convenience. Interestingly, the global rating reflected efficacy more than side effects. In B, patients scored themselves reasonably well with regard to symptoms, physical functioning, and quality of life. There did not appear to be changes in either treatment satisfaction or quality-of-life dimensions during the 12-month period. 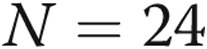 at baseline;
at baseline; 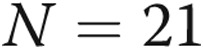 at week 52.
at week 52.
