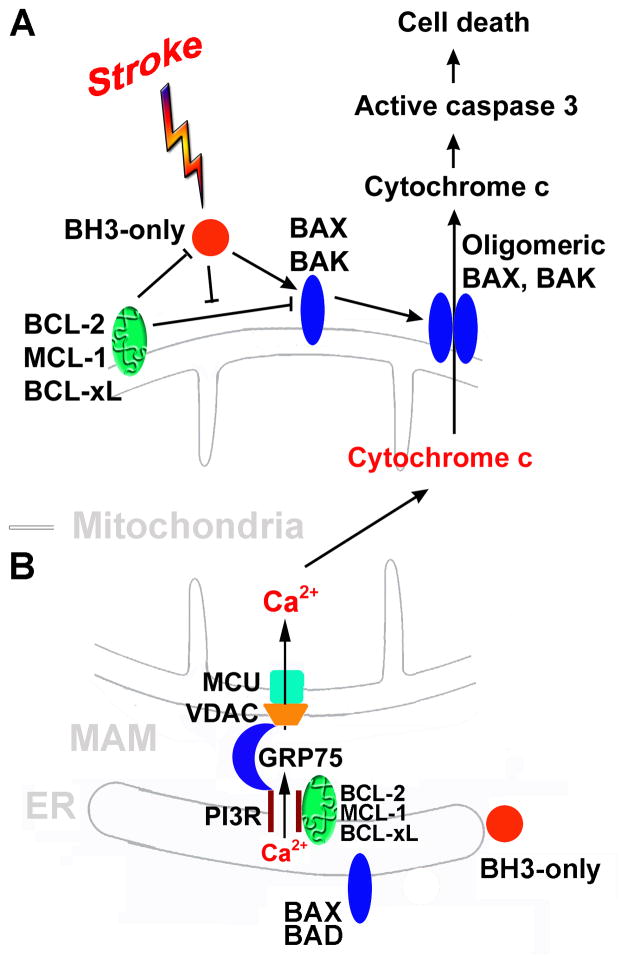
Fig. 2.
BCL-2 family-mediated cell death. A. The BCL-2 family regulates mitochondria-dependent apoptosis. BH3-only family members act as sentinels for many death stimuli including ischemia. They can mediate the activation of the multidomain pro-apoptotic BAX and BAK allowing oligomerization. Upon BAX and BAK oligomerization, the mitochondrial outer membrane is permeabilized releasing a apoptogenic proteins from the mitochondrial intermembrane space, such as cytochrome c, into the cytosol. Released cytochrome c triggers the activation of a downstream caspase cascade leading to cell death. BH3-only proteins can be sequestered by anti-apoptotic BCL-2 family members (e.g. BCL-2, MCL-1, BCL-xL,, etc.). B. The BCL-2 family regulates ER-mitochondria Ca2+ crosstalk at the MAM. The basic structure for release of Ca2+ at MAM is the IP3R on the ER, and VDAC and MCU on the mitochondrion. IP3R and VDAC are physically coupled by the chaperone GRP75/mortalin. Excessive increases in mitochondrial matrix Ca2+ triggers opening of the mitochondrial outer membrane permeability pore causing the release of cytochrome c and other pro-apoptotic factors into the cytoplasm. ER: endoplasmic reticulum; GRP75: glucose-regulated protein 75/mortalin; IP3R: inositol trisphosphate receptor; MAM: mitochondria-associated ER membrane; MCU: mitochondrial Ca2+uniporter; VDAC: voltage-dependent anion channel.