Abstract
Tetrabromobisphenol A (TBBPA) is the brominated flame retardant with the largest production volume worldwide. NTP 2-year bioassays found TBBPA dose-dependent increases in uterine tumors in female Wistar Han rats; evidence of reproductive tissues carcinogenicity was equivocal in male rats. To explain this apparent sex-dependence, the disposition and toxicokinetic profile of TBBPA were investigated using female Wistar Han rats, as no data were available for female rats. In these studies, the primary route of elimination following [14C]-TBBPA administration (25, 250 or 1000 mg/kg) was in feces; recoveries in 72 h were 95.7 ± 3.5%, 94.3 ± 3.6% and 98.8 ± 2.2%, respectively (urine: 0.2–2%; tissues: <0.1). TBBPA was conjugated to mono-glucuronide and -sulfate metabolites and eliminated in the bile. Plasma toxicokinetic parameters for a 250 mg/kg dose were estimated based on free TBBPA, as determined by UV/radiometric-HPLC analyses. Oral dosing by gavage (250 mg/kg) resulted in a rapid absorption of compound into the systemic circulation with an observed Cmax at 1.5 h post-dose followed by a prolonged terminal phase. TBBPA concentrations in plasma decreased rapidly after an IV dose (25 mg/kg) followed by a long elimination phase. These results indicate low systemic bioavailability (F < 0.05), similar to previous reports using male rats. Elimination pathways appeared to become saturated leading to delayed excretion after a single oral administration of the highest dose (1000 mg/kg); no such saturation or delay was detected at lower doses. Chronic high exposures to TBBPA may result in competition for metabolism with endogenous substrates in extrahepatic tissues (e.g., SULT1E1 estrogen sulfation) resulting in endocrine disruption.
Chemical compounds studied in this article: Tetrabromobisphenol A (PubChem CID: 6618)
Keywords: Disposition, Tetrabromobisphenol A, TBBPA, Brominated flame retardant, Persistent organic pollutants, Toxicokinetics, Disposition, Female rat
1. Introduction
Tetrabromobisphenol A (TBBPA, 2,2′,6,6′-Tetrabromo-4,4′-isopropylidene diphenol; CAS No. 79-94-7) is the largest production volume brominated flame retardant (BFR) with >230,000 tons manufactured per year [13]. This represents ∼60% of all worldwide demand for BFR chemicals; TBBPA is used primarily as a reactive (chemically-bound) flame retardant in >90% of printed circuit boards, as well as in laminates, paper, textiles, as a plasticizer, and intermediate for the syntheses of other flame retardants [7]. Although it has been purported to be sequestered in its end-use product [7], TBBPA has been consistently detected in environmental samples [12]. Use of TBBPA as an additive flame retardant (not chemically bound) has been reported in products like Acrylonitrile–Butadiene–Styrene plastic casings and this application is expected to increase as other flame retardants (e.g., high molecular weight polybrominated diphenyl ethers and hexabromocyclododecanes) are withdrawn from the market [8]. This additive use increases the potential for its release to the environment. Recent studies of nursing mothers found greater than 50% of breast milk samples and 30% of maternal/cord serum samples contained detectable levels of TBBPA, demonstrating widespread exposure to mothers & fetuses and potentially to newborns via breastfeeding [1], [3], [9], [10], [35], [36].
TBBPA has been shown to be a ligand for several hormone receptors in vitro. TBBPA is a T4 competitor in the TTR-binding assay [19], [20] and binds to the rat thyroid hormone receptor with high affinity [25]. TBBPA was also reported to bind to human transthyretin [28]. Both TBBPA and TBBPA-sulfate have been shown to be human peroxisome proliferator-activated receptor γ [31], [32]. In studies using the estrogen-dependent rat pituitary tumor cell line MtT/E-2, TBBPA enhanced proliferation but to a lower extent than its non-brominated analog, bisphenol A [25]. TBBPA binds to the estrogen receptor, but to a lower degree than bisphenol A [33]. TBBPA was an estrogen receptor subtype alpha (ERα) agonist and a progesterone receptor (PR) antagonist in transfected yeast. TBBPA and two metabolites (2,6-dibromo-4-[2-hydroxypropane-2-yl]-phenol and 2,6-dibromo-4-[2-methoxypropane-2-yl]-phenol) have been shown to have estrogenic activity in MCF-7 cells [26], [33], [39]. TBBPA has been shown to be an inhibitor of estradiol sulfation in vitro [19]. Recent crystallographic studies show that it can be bound with high affinity to the estrogen sulfotransferase SULT1E1 [16].
TBBPA has an LD50 of greater than 5 g/kg when administered as a single dose by gavage to rats [21]. Intraperitoneal administration of TBBPA has been shown to cause hepatotoxicity and heme metabolism disturbances [37], [38], phenomena that may relate to the formation of free radicals in vivo [11]. In repeat-dose subacute and one-generation reproductive studies, TBBPA exposures resulted in decreased thyroxine levels and other endocrine effects [40]. Hakk et al. [18] demonstrated that, at a dose of 2 mg/kg, TBBPA is readily absorbed from the gastrointestinal tract of male Sprague Dawley (SD) rats where it undergoes biotransformation to o-glucuronide and o-sulfate conjugates followed by biliary elimination. TBBPA was eliminated in the feces as parent compound demonstrating intestinal deconjugation. Similarly, at a 300 mg/kg dose of TBBPA to male SD rats, TBBPA was rapidly absorbed with a plasma Cmax of 103 μmol/L reached within 3 h; TBBPA-sulfate was the major metabolite detected in plasma along with TBBPA-glucuronide [34]. Kang et al. [24] reported plasma Cmax values of 23, 34, and 57 μmol/L for oral doses of 200, 500, and 1000 mg/kg TBBPA (Tmax occurred at 4.2–5 h), respectively for male SD rats; metabolites were not described. Kuester et al. [27] demonstrated that at a 20 mg/kg dose of TBBPA in male Fischer-344 (F-344) rats, the systemic bioavailability of the compound was low (1.6%). The bioavailability of TBBPA in humans is unknown, although it is expected to be low. TBBPA was absorbed from the gut of healthy human volunteers receiving a single oral dose of 0.1 mg/kg but TBBPA was below the limit of detection in all blood samples. However, a glucuronide conjugate of TBBPA was present in samples collected at all of the time points up to 72 h, with peak concentrations detected between 2 and 6 h and traces of TBBPA-glucuronide were detected in urine [34]. TBBPA was detected at low levels (<1–3.4 pmol/g lipid) in serum of computer workers and workers in an electronics dismantling plant [17], [22]. TBBPA was metabolized by human liver subcellular fractions in a similar fashion to those of rats [41].
Ongoing analyses of data from TBBPA chronic exposure studies has demonstrated that TBBPA induced highly malignant uterine tumors in female Wistar Han [Crl:WI(Han)] rats in a dose-dependent manner. Male Crl:WI(Han) rats did not develop a significant tumor burden whereas administration of TBBPA resulted in increased incidences of nonneoplastic lesions of the liver and kidney in male B6C3F1 mice and in the forestomach of male and female mice [30]. However, no disposition, metabolism or kinetics data are available for the female Crl:WI(Han) rat. Therefore, studies were conducted to characterize the disposition of TBBPA in female Crl:WI(Han) rats following single or repeated oral or intravenous administration. The toxicokinetic profile of TBBPA in plasma after a 250 mg/kg dose was also investigated, as this dose was the lowest dose found to be associated with tumor formation in the NTP 2-year bioassay [30].
2. Materials and methods
2.1. Chemicals
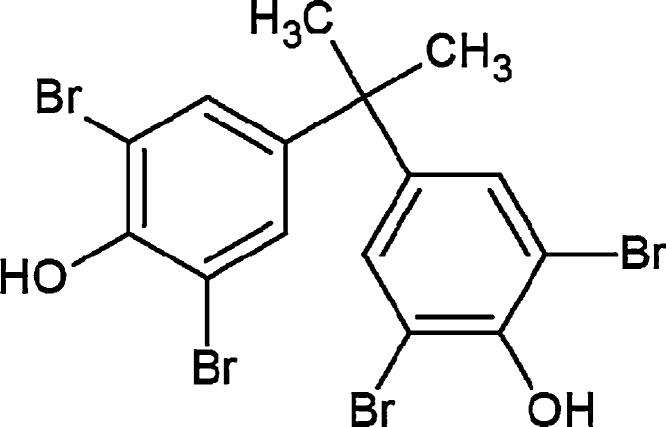
[14C]-Radiolabeled TBBPA (uniform ring-labeled; Fig. 1), previously described by Kuester et al. [27] was generously provided by I. Glenn Sipes at the University of Arizona (Tucson, AZ) for use in the present study. Reversed-phase HPLC fractionation (described below) was used to acquire a radiochemical purity of >98% (specific activity = 90.3 mCi/mmol). [14C]-TBBPA was reconstituted in dimethyl sulfoxide. Chemical purity was determined to be >98% as compared to a TBBPA reference standard (Sigma–Aldrich; St. Louis, MO). Scintillation cocktails were obtained from MP Biomedicals (Ecolume; Santa Ana, CA) or Perkin-Elmer (Ultima-Flo M & PermaFluor E+; Torrance, CA). Cremophore EL®, dimethylsulfoxide, β-glucuronidase (EC 3.2.1.31, Type B-10), ammonium acetate, d-saccharic acid 1,4-lactone, and aryl sulfatase were purchased from Sigma–Aldrich. All other reagents used in these studies were high performance liquid chromatography (HPLC) or analytical grade.
Fig. 1.
Chemical structure of TBBPA.
2.2. Animal model
Female Crl:WI(Han) rats (11 weeks, ∼200 g at time of dosing; Charles River, Raleigh NC) were used in these studies. Animals were maintained in an AAALAC-approved animal care facility for a minimum of 7 days in plastic shoebox cages prior to dosing. Animals were housed individually in plastic metabolism cages for separate collection of urine and feces. Food (NIH #31) and water were provided for ad libitum consumption. All procedures were approved by the NIEHS Institutional Care and Use committee.
2.3. Dosing
Animals were administered a single dose of TBBPA by gavage or by intravenous (IV) bolus through an indwelling catheter. Dosing solutions were formulated to administer ∼50 μCi/kg/rat and contained amounts of non-radiolabeled TBBPA for delivery of doses up to 1000 mg/kg. Oral doses were: 25, 250 (4 mL/kg) or 1000 mg/kg (8 mL/kg) and the IV dose was administered at a dose level of 25 mg/kg (1 mL/kg). Dosing vehicle was ethanol, water, and an emulsifying agent (Cremophore EL®) in a 1:3:1 ratio. Dosing solutions contained 2% or less DMSO.
2.4. Sample collections
Following administration of the compound (N = 3–6/treatment group) excreta and cage rinses were collected at 6, 12, 24, 48, and 72 h. Animals were euthanized by CO2 asphyxiation. Tissues (adipose, adrenals, brain, heart, kidneys, large intestine & contents, liver, lung, muscle, pancreas, ovaries, skin, small intestine & contents, spleen, stomach & contents, thymus, thyroid, urinary bladder, and uterus) were collected at necropsy and stored at −80 °C until analysis. Blood samples were collected via cardiac puncture at the time of death. Animals used in kinetic studies (250 mg/kg oral and 25 mg/kg IV) contained an indwelling jugular vein cannula from which blood (∼150 μL) was obtained using heparinized syringes at 7.5 min, 15 min, 30 min, 1 h, 3 h, 6 h, 12 h, and 24 h post-dose. Withdrawn blood volumes were replaced with equal amounts of saline. One group of rats with an indwelling common bile-duct cannula was used to determine biliary elimination of TBBPA metabolites following a 250 mg/kg dose. Animals were surgically altered by the vendor (Charles River) and were fully recovered from anesthesia before delivery (approx. 24 h before dosing). Bile was collected continuously and analyzed at 1 h intervals up to 6 h post-dose. Samples were placed in labeled pre-weighed vials after all collections and maintained at −80 °C until analyses. Plasma was isolated from heparinized blood by centrifugation (5 min at 3000 RPM). Bile samples (∼100 μL) were diluted with 1 mL water prior to analyses. Total mass of incompletely necropsied tissues were calculated based on percentages of final body weight (adipose: 11%, muscle: 50%, skin: 16%) using previously published values [6]. All other tissues were completely collected and weighed gravimetrically.
2.5. Analytical methods
Samples were analyzed in parallel for quantitative and qualitative analyses. Quantitative analyses of total [14C]-radioactivity content was determined using a Beckman Coulter (Brea, CA) LS6500 Liquid Scintillation Counter (LSC). Total [14C]-radioactivity content of bile (100 μL), plasma (10 μL), urine (10–100 μL) and cage rinses (1 mL) was assayed in triplicate with the LSC. Fecal samples were air dried in a fume hood, weighed and ground to a powder using a mortar and pestle. Triplicate aliquots of feces and tissues (∼25 mg) were weighed and [14C]-radioactivity was quantified following combustion in a Packard 307 Biological Sample Oxidizer by LSC analysis.
To determine the nature of phase II metabolites of TBBPA in bile, samples were incubated at 55 °C for 60 min with β-glucuronidase (5000 U/mL) or aryl-sulfatase (11 U/mL) as described by Andersen et al. [2]. Bile incubated with buffer (200 μM ammonium acetate in water, pH 5.0) was incubated under identical conditions and served as controls. Reactions were terminated by rapidly cooling the samples to −20 °C followed by HPLC-radiometric analyses.
For plasma toxicokinetic analyses, TBBPA was quantified by UV/Vis absorbance at 210 nm and radiochemical detection following HPLC separation. [14C]-TBBPA was purified using an HPLC system composed of a Waters 1525 binary HPLC pump, Phenomenex Luna 5 μ C18(2) 250 × 4.6 mm column (Phenomenex, Torrance CA), and a Waters 2487 dual λ absorbance detector with an in-line IN/US β-RAM model 3 Flow Scintillation Analyzer (LabLogic, Inc., Brandon FL). The HPLC system used for analysis of biological samples was composed of a Waters 2695 Separations Module, Agilent Eclipse Plus C18 column (3.5 μm, 4.6 mm × 150 mm), and a Waters 996 Photodiode Array with an in-line Radiomatic 500TR Flow Scintillation Analyzer. Mobile phases consisted of 0.1% trifluoroacetic acid in water (mobile phase A) and 0.1% trifluoroacetic acid in acetonitrile (mobile phase B). Sample separations were performed using a gradient; initial conditions (60% A) were maintained for 5 min; A was then reduced to 10% over 2 min then to 0% A over 13 min. The column was returned to initial conditions and allowed to equilibrate for 5 min before re-use. Flow rates were 1 ml/min. Instrument control and analysis software were Empower Pro (Waters Corp.) and FLO-ONE for Windows (Packard Instruments Co., Inc.; v. 3.6). TBBPA was quantified based on a 5-point calibration curve.
Biliary metabolites were further characterized by LC/MS analyses. The LC/MS consisted of an Agilent 1100 HPLC (Agilent Technologies, Santa Clara CA) and a ThermoFinnigan LCQ AdvantageMax mass spectrometer (Thermo Scientific, Waltham MA) equipped with an ESI source. Trap conditions were similar to those described by Kang et al. [23]. Conditions were: sheath gas = N2 at 20 L/min, capillary temperature = 275 °C, spray voltage = 5 kV, capillary voltage = −33 V, tube lens offset = −30 V. The collision gas was Helium and ions were isolated over a mass window of 5 Da for tandem mass spectrometric studies. HPLC mobile phases consisted of 100% acetonitrile (A) and 100% water (B) using isocratic conditions (A:B, 70:30) with a flow rate of 200 μL/min. Bile samples were fractionated prior to MS analysis using the Waters HPLC system and fractions collected between 1–3 min and 7–9 min were infused into the LC/MS using a syringe pump. Following confirmation of the mass of TBBPA (543 Da), full mass spectrometry (MS) scans were performed followed by manual selection of the most abundant ions for isolation and fragmentation.
2.6. Pharmacokinetic modeling
Free TBBPA in plasma collected from individual animals at each time point described above (determined by UV/radiometric-HPLC) was used to construct a time–concentration data table for each animal. These data were fit to established pharmacokinetic models using the Phoenix WinNonlin (Certara USA, Inc.; St. Louis, MO) software package. Time–concentration data from animals administered TBBPA intravenously were fit to a one-compartment model with bolus input and first order output. Time–concentration data from animals administered TBBPA by gavage were fit to a one-compartment model with first order input and output or a two-compartment model with first order input and output. Final distribution/elimination phases were assessed by non-compartmental analysis based on observed deviations from concentrations predicted by compartmental analyses. Goodness of fit was assessed by comparing the sum of squared residuals.
3. Results
3.1. Distribution and elimination studies
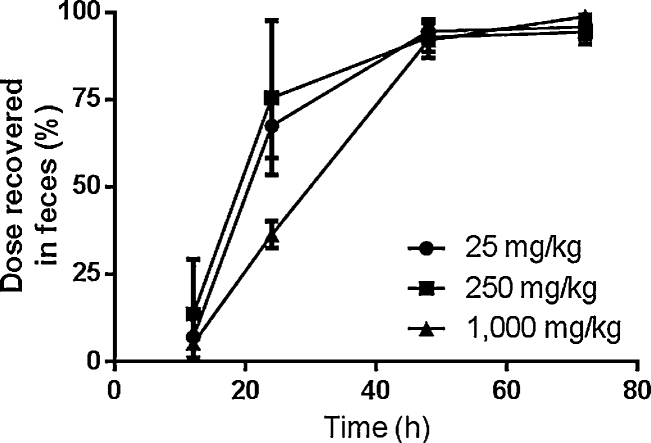
The primary route of elimination of radioactivity was in feces following oral administration of [14C]-TBBPA; dose recoveries in 72 h were 95.7 ± 3.5%, 94.3 ± 3.6% and 98.8 ± 2.2%, for the 25, 250, and 1000 mg/kg dose, respectively (Fig. 2). Animals administered 1000 mg/kg of TBBPA showed a clear delay in rates of fecal elimination through 24 h. Urine contained from 0.2–2% of dose. Less than 0.1% of the administered [14C]-radioactivity was detected in tissues collected at 72 h following oral doses. In addition, four animals were administered TBBPA (250 mg/kg) for 5 days to determine the effects of repeated exposure on elimination and distribution. Four doses of non-radiolabeled TBBPA were followed by a single dose of [14C]-labeled TBBPA (50 μCi/kg) and animals were euthanized 24 h after the final dose. Recoveries were similar to those found following a single dose, with 84 ± 8% of the terminal dose recovered from feces (76 ± 22% was recovered by 24 h in single-dose studies). Less than 1% of the dose was recovered in urine and tissue retention was similarly minimal.
Fig. 2.
Cumulative elimination following oral administration of TBBPA.
The amount of administered [14C]-radioactivity in selected tissues was estimated at 1, 3, 6, 24, and 72 h following oral administration of [14C]-TBBPA (250 mg/kg, 50 μCi/kg). The vast majority of recovered [14C]-radioactivity was found in the gastrointestinal tract contents at 1, 3, and 6 h (Table 1). Radioactivity in blood accounted for 1–8% of the dose at these early time points. Tissues contained little radioactivity at 24 h (<2%), with the majority of the unrecovered radioactivity present in the large intestine contents at this time point. Small but measurable amounts of TBBPA-derived radioactivity remained in the liver, muscle, skin, and small intestine, as well as the small and large intestinal contents at 72 h following oral doses of 25, 250, or 1000 mg/kg (Table 2). Adrenals, brain, heart, ovaries, spleen, thymus, thyroid, urinary bladder, and uterus retained less that 0.01% of administered doses.
Table 1.
Disposition of [14C]-radioactivity following oral administration of TBBPA: 250 mg/kg, 1–24 h.
| 1 h (N = 4) |
3 h (N = 4) |
6 h (N = 4) |
24 h (N = 4) |
|||||
|---|---|---|---|---|---|---|---|---|
| % dose | (nmol-eq/g tissue) | % dose | (nmol-eq/g tissue) | % dose | (nmol-eq/g tissue) | % dose | (nmol-eq/g tissue) | |
| Mean ± S.D. | (Mean ± S.D.) | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | (Mean ± S.D. | Mean ± S.D. | (Mean ± S.D.) | |
| Adipose | 0.3 ± 0.1 | (13 ± 3) | 0.5 ± 0.1 | (22 ± 6) | 2 ± 2 | (91 ± 58) | 0.1 ± 0.08 | (4 ± 3) |
| Kidneys | 0.1 ± 0.03 | (30 ± 15) | 0.04 ± 0.02 | (24 ± 11) | 0.7 ± 0.3 | (388 ± 131) | <0.01 | (2 ± 1) |
| Large intestine | 0.1 ± 0.1 | (78 ± 49) | 0.3 ± 0.2 | (143 ± 79) | 0.4 ± 0.1 | (203 ± 41) | 0.3 ± 0.1 | (149 ± 55) |
| Large intestine contents | 0.2 ± 0.1 | (62 ± 32) | 9 ± 6 | (2290 ± 1412) | 1 ± 0.7 | (451 ± 230) | 23 ± 19 | (3378 ± 2973) |
| Liver | 2 ± 0.7 | (212 ± 72) | 2 ± 0.6 | (179 ± 93) | 3 ± 2 | (312 ± 166) | 0.2 ± 0.1 | (20 ± 9) |
| Lung | <0.01 | (29 ± 12) | 0.02 ± 0.01 | 15 ± 7) | (0.4 ± 0.2 | (234 ± 102) | <0.01 | (2 ± 1) |
| Muscle | 0.5 ± 0.2 | (4 ± 2) | 0.5 ± 0.4 | (4 ± 3) | 3 ± 2 | (36 ± 8) | 0.1 ± 0.04 | (1 ± 0.3) |
| Pancreas | 1 ± 0.7 | (339 ± 198) | 0.4 ± 0.4 | (131 ± 96) | 0.5 ± 0.1 | (195 ± 53) | 0.1 ± 0.05 | (24 ± 21) |
| Skin | 1 ± 1 | (30 ± 40) | 0.4 ± 0.1 | (11 ± 4) | 5 ± 4 | (171 ± 50) | 0.1 ± 0.04 | (2 ± 1) |
| Small intestine | 1 ± 0.7 | (452 ± 196) | 3 ± 2 | (887 ± 457) | 1 ± 0.6 | (284 ± 105) | 0.4 ± 0.2 | (142 ± 79) |
| Small intestine contents | 23 ± 16 | (3806 ± 2383) | 50 ± 4 | (9275 ± 1171) | 9 ± 3 | (2007 ± 537) | 2 ± 0.8 | (357 ± 167) |
| Stomach | 3 ± 1 | (2400 ± 954) | 2 ± 0.9 | (855 ± 93) | 3 ± 0.8 | (1704 ± 652) | 0.04 ± 0.04 | (29 ± 30) |
| Stomach contents | 74 ± 13 | (19,463 ± 1868) | 26 ± 8 | (11,460 ± 7113) | 36 ± 21 | (12,822 ± 5467) | 0.1 ± 0.1 | (331 ± 453) |
| Feces | 0.02 ± 0.01 | 0.01 ± 0.02 | 0.1 ± 0.1 | 82 ± 18 | ||||
| Urine | n.d. | 0.5 ± 0.1 | 1.2 ± 0.7 | 0.3 ± 0.1 | ||||
| Blood | 0.6 ± 0.2 | 0.4 ± 0.2 | 8 ± 0.03 | 0.06 ± 0.05 | ||||
| Bile | n.d. | n.d. | 6 ± 7 | n.d. | ||||
n.d.: not determined.
Table 2.
Disposition of [14C]-radioactivity following oral administration of TBBPA: 25, 250, or 1000 mg/kg at 72 h.
| 25 mg/kg, 72 h (N = 4) |
250 mg/kg, 72 h (N = 4) |
1000 mg/kg, 72 h (N = 4) |
||||
|---|---|---|---|---|---|---|
| % dose | (nmol-eq/g tissue) | % dose | (nmol-eq/g tissue) | % dose | (nmol-eq/g tissue) | |
| Mean ± S.D. | (Mean ± S.D.) | Mean ± S.D. | (Mean ± S.D.) | Mean ± S.D. | (Mean ± S.D.) | |
| Large intestine contents | 0.02 ± 0.03 | (0.5 ± 0.6) | 0.02 ± 0.01 | (21 ± 32) | 0.4 ± 0.3 | (241 ± 169) |
| Small intestine contents | <0.01 | (0.07 ± 0.08) | 0.01 ± 0.01 | (3 ± 3) | 0.08 ± 0.06 | (63 ± 50) |
| Liver | 0.01 ± 0.002 | (0.1 ± 0.01) | 0.02 ± 0.01 | (2 ± 1) | 0.04 ± 0.01 | (17 ± 5) |
| Small intestine | <0.01 | (0.05 ± 0.04) | <0.01 | (1 ± 1) | 0.01 ± 0.01 | (14 ± 6) |
| Muscle | 0.02 ± 0.03 | (0.02 ± 0.02) | <0.01 | (0.2 ± 0.2) | 0.03 ± 0.02 | (1 ± 0.8) |
| Skin | 0.01 ± 0.01 | (0.02 ± 0.02) | <0.01 | (3 ± 5) | 0.01 ± 0.01 | (1 ± 0.7) |
A set of animals received TBBPA by IV administration to enhance interpretation of oral absorption data. At 6 h post-dosing (25 mg/kg, 50 μCi/kg) [14C]-radioactivity was present in the large intestine (3.2 ± 3%), small intestine (3.2 ± 3.1%), liver (1.8 ± 0.6%), and adipose (1 ± 0.8%) tissues; all other tissues retained less than 1% of the administered dose at 6 h post-dose. Approx. 10% and 1% of the administered radioactivity was eliminated in the feces and urine, respectively, within 6 h. The remainder of the dose was recovered in the contents of the small and large intestines (approx. 15% and 61% at 6 h post dose, respectively). Conversely, animals administered 250 mg/kg by gavage eliminated 1–12% of the administered radioactivity into bile in 6 h, while 36% remained in the stomach and 9% was recovered from small intestine contents (1% in large intestine contents); approx. 8% of the dose was found in the blood, consistent with plasma toxicokinetic studies described below. These data support the conclusion that TBBPA is cleared from the systemic compartment via bile for fecal elimination.
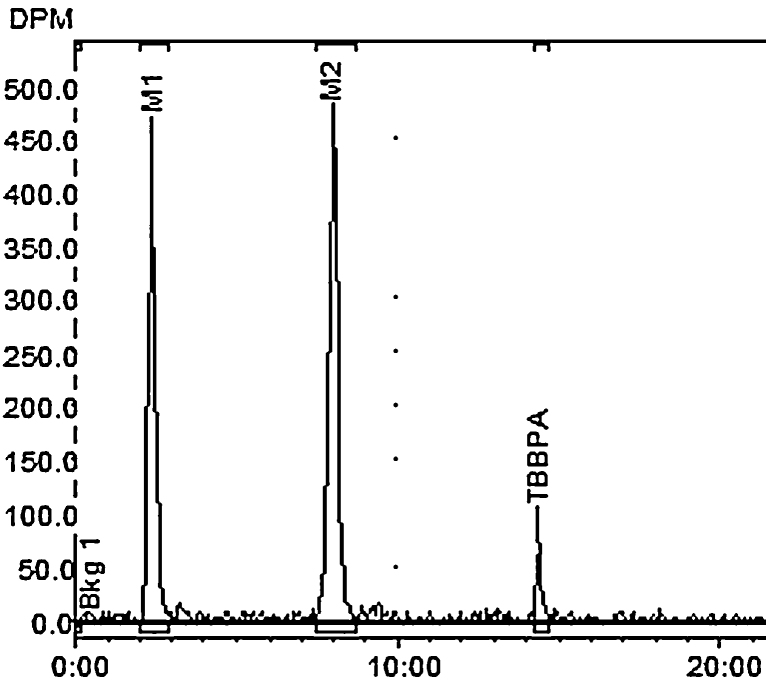
Two major [14C]-radiolabeled metabolite peaks and a small amount of parent TBBPA were detected in bile of the rats receiving 250 mg/kg by gavage (Rt = 2 and 8 min respectively; Rt for [14C]-TBBPA = 14.5 min; Fig. 3). Incubation with β-glucuronidase resulted in increased detection of parent compound with stoichiometric reductions of metabolite 2 (M2). Incubation with sulfatase resulted in the disappearance of metabolite 1 (M1) and a reduction in the recovery of M2. Parent peak area increased with both enzymatic treatments. Residual β-glucuronidase activity in the sulfatase reaction media likely accounts for the loss of M2.
Fig. 3.
Representative HPLC-radiometric chromatograms of TBBPA metabolites in bile after oral administration of [14C]-TBBPA (250 mg/kg, PO).
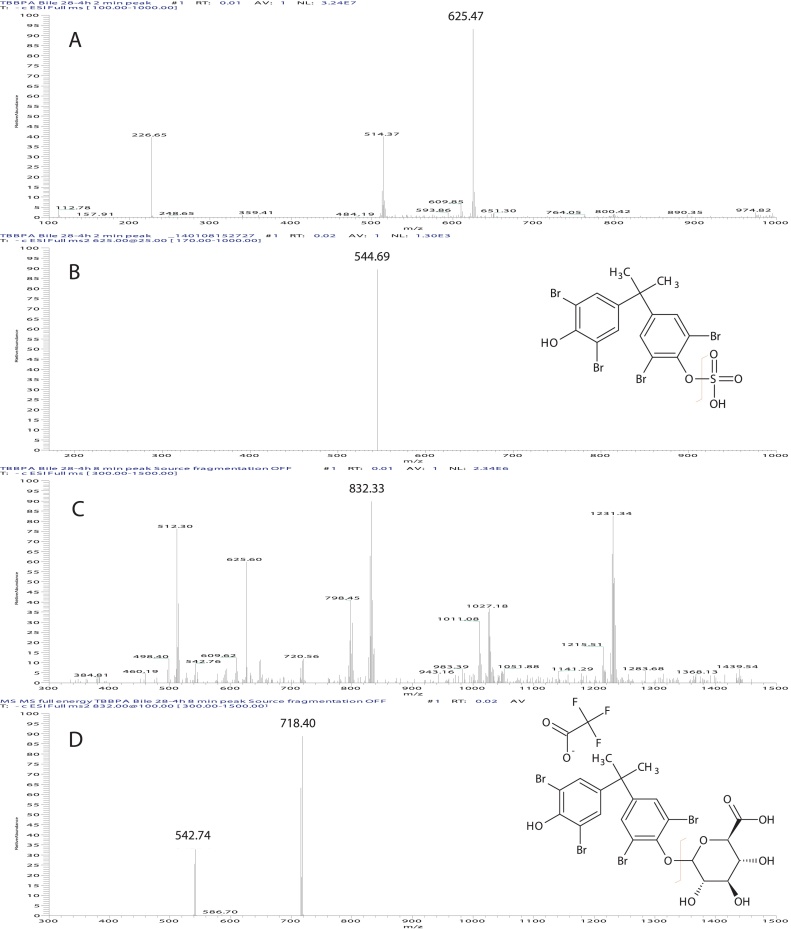
M1 and M2 were isolated from bile using the HPLC method described above and were subjected to electrospray mass spectral analyses. Infusion of the M1 fraction (Rt = 2 min) showed a major ion with m/z = 625 (Fig. 4, panel A). Collision induced fragmentation of this ion resulted the loss of 82 Da (consistent with the mass of a sulfuryl group) and formation a daughter ion with m/z = 543. Infusion of the M2 fraction (Rt = 8 min) resulted in a major ion with m/z = 832 (Fig. 4, panel B). Collision induced fragmentation of this ion resulted in the loss of 114 Da (trifluouracetic acid adduct) and formation a product ion with m/z = 718. This ion was further fragmented resulting in the loss of 175 Da (glucuronic acid) with a resulting ion with m/z = 543. As such, M1 has been identified as TBBPA-sulfate and M2 as TBBPA-glucuronide.
Fig. 4.
Electrospray mass spectrum of ([M−H]− ion) of A: TBBPA biliary metabolite peak 1 (TBBPA-sulfate); B: collision induced fragmentation of m/z 625 gives a loss of 80 amu); C: TBBPA biliary metabolite peak 2 (TFA-adducted TBBPA-glucuronide); D: collision induced fragmentation of m/z 832 & 719 gives a loss of 114 and 176 amu, respectively).
3.2. Plasma toxicokinetic studies
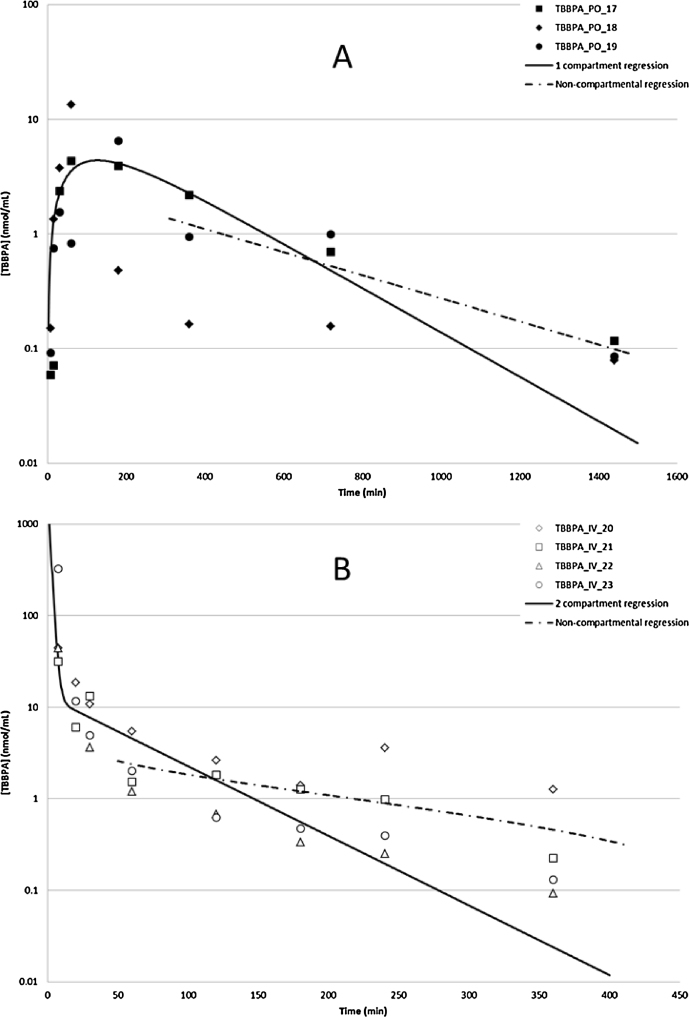
Toxicokinetic profiles for TBBPA in plasma following oral or IV administrations were estimated based on free TBBPA detected in plasma (Fig. 5, panel A). Oral administration of TBBPA (250 mg/kg) resulted in a rapid absorption of compound, with a mean Cmax of 5.4 μmol/L occurring at 127 min post-dose and the decline in plasma concentration consistent with a one-compartment model. The absorption and elimination half-lives were ∼54 and 155 min, respectively. When TBBPA was given by IV administration (25 mg/kg), plasma concentrations rapidly decreased, with ∼2% of the administered radioactivity remaining at 6 h post dose (Fig. 5, panel B). The time–concentration profile for free TBBPA in plasma following IV administration of [14C]-TBBPA was largely consistent with a two-compartment model (distribution half-life = 2 min; terminal half-life = 22 min). However, the observed goodness of fit at late time-points was sub-optimal, especially at the terminal time-points, resulting in overestimation of clearance rates for TBBPA from the central compartment. To better estimate clearance, data were subjected to non-compartmental analyses whereupon the terminal half-life following oral administration of TBBPA was ∼290 min; following IV, 133 min. Taken together, these results show there is less than 5% systematic bioavailability for TBBPA in female Crl:WI(Han) rats (Table 3).
Fig. 5.
Time–concentration profiles of TBBPA in plasma following A: oral administration, 5 min-24 h (250 mg/kg; N = 3); B: IV administration, 5 min-6 h (25 mg/kg; N = 4). Regression lines show model-predicted data. TBBPA was quantified by UV-detection at 210 nm following separation by HPLC (see Section 2).
Table 3.
TBPA bioavailability parameters in female Crl:WI(Han) rats.
| Dose | AUC | F | |
|---|---|---|---|
| (nmol) | (min*nmol/mL) | (%) | |
| IV (25 mg/kg) | 8580 | 4286 | 4.8% |
| Oral (250 mg/kg) | 72,323 | 1729 |
4. Discussion
Female Crl:WI(Han) rats exhibited similar ADME/TK characteristics as those described for male F-344 [27] and male SD rats [18], [24], [34]. TBBPA appeared to have low systemic bioavailability due to a significant first pass effect in the liver, with biliary elimination leading to fecal excretion at all does examined and after repeated administrations of 250 mg/kg. Plasma levels of TBBPA described here were up to 3 orders of magnitude higher than those observed in serum from occupationally-exposed humans. Nevertheless, these data may be useful in describing the upper bounds of exposure for conservative risk assessments of TBBPA exposures.
The results presented here, along with data from other studies, indicate that TBBPA is absorbed from the gastrointestinal tract following oral administration to female Crl:WI(Han) rats. Significant first pass metabolism was likely and rapid elimination of an IV-administered dose into the gut contents and feces supported this conclusion. Low levels of parent compound did persist in the free fraction of plasma for up to 24 h, indicating a possible route for low level on-going exposure to tissues such as the uterus. Entero-hepatic cycling following deconjugation by gut microflora [5] and/or action by tissue glucuronidases [4] may play a role in preserving this persistent low level of TBBPA. Additionally, individuals deficient in specific conjugation capacities, esp. fasting and frail adults [14], [29] as well as infants and neonates [15] may be exposed to higher systemic levels, especially at high doses of TBBPA. Additionally, as TBBPA is being used more extensively as an additive BFR in consumer products, it is expected that higher continuous exposures will be experienced by more diverse and sensitive populations.
Low but detectable amounts of TBBPA persisting in blood at the 12 and 24 h time points could not be accounted for in the simple one-compartment model and inter-individual variation (Crl:WI[Han] rats are an outbred strain) precluded meaningful model fit to a 2-compartment oral model. This persistence of compound in plasma may be due, at least in part, to entero-hepatic circulation of TBBPA. Notably, biliary elimination of [14C]-radioactivity was appreciably lower at 250 mg/kg in fed female Crl:WI(Han) rats, with 12% or less of the dose eliminated in bile in 6 h following 250 mg/kg oral administration of TBBPA, as compared with ∼50% of dose recovered in bile in 2 h following a 20 mg/kg dose administration to fasted F-344 male rats [27]. Saturation of absorption, delayed gastric emptying, or binding to food particles may have contributed to the apparent increase in retention of compound in the stomach contents. Tissue distribution was minimal, with appreciable (>1% of dose) being recovered in GI tract contents rather than within tissues at all time-points, further supporting a major role for hepatic detoxification and elimination of TBBPA in vivo even after repeated administrations. Evidence from short-term (1–6 h) exposure studies suggest biliary elimination of TBBPA-derived material is slower in non-fasted female Crl:WI(Han) rats than has been previously reported using other strains. However, these data indicate that TBBPA has a similar ADME-TK profile in female Crl:WI(Han) rats as that previously reported for male SD or F-344 rats [18], [27].
Doses selected for the studies were based on those calculated as a function of maximum tolerated dose rather than as a function of occupational exposures and were analogous to those used in the National Toxicology Program 2-year cancer bioassay [30]. There was no evidence of preferential distribution or retention of TBBPA in uterus leading to the conclusion that the dispositional fate of TBBPA did not appear to have a direct linkage to TBBPA-dose dependent uterine carcinogenicities. To that end, uterine carcinogenesis seen in female Crl:WI(Han) rats following TBBPA exposure may be due to indirect endocrine disruption; there is emerging evidence that TBBPA disrupts estrogen metabolism by competing for access to tissue-specific sulfotransferases, e.g. SULT1E1 [16]. Alterations in estrogen signaling pathways by chronic exposure to high levels of TBBPA may contribute to the cellular events necessary for the uterine neoplasia demonstrated in the 2-year cancer bioassay of TBBPA [30]. Long term exposures may be needed before treatment-related effects are manifested. It is critical to note that the uterine carcinogenesis see in female Crl:WI(Han) rats is due to indirect endocrine disruption, as shown in high dose animals studies as well as in vitro data, with unknown relevance to humans exposed at environmental levels via plausible routes of exposure. Additional studies are being conducted to delineate endocrine, cellular, proteomic and/or genomic changes associated with TBBPA-dependent carcinogenicity in rodents.
Conflicts of interest
None declared.
Funding
This research was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health [Project ZIA BC 011476].
Acknowledgements
The authors would like to thank Dr. June Dunnick (National Toxicology Program) for advice and Ms. Sherry Coulter for technical assistance.
Footnotes
Available online 14 May 2014Chemical
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.03.005.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Abdallah M.A., Harrad S. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: relationship to external exposure. Environ. Int. 2011;37:443–448. doi: 10.1016/j.envint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Andersen A., Holte H., Slordal L. Pharmacokinetics and metabolism of doxorubicin after short-term infusions in lymphoma patients. Cancer Chemother. Pharmacol. 1999;44:422–426. doi: 10.1007/s002800050999. [DOI] [PubMed] [Google Scholar]
- 3.Antignac J.P., Cariou R., Maume D., Marchand P., Monteau F., Zalko D., Berrebi A., Cravedi J.P., Andre F., Le Bizec B. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Mol. Nutr. Food Res. 2008;52:258–265. doi: 10.1002/mnfr.200700077. [DOI] [PubMed] [Google Scholar]
- 4.Antunes I.F., Haisma H.J., Elsinga P.H., van Waarde A., Willemsen A.T., Dierckx R.A., de Vries E.F. In vivo evaluation of 1-O-(4-(2-fluoroethyl-carbamoyloxymethyl)-2-nitrophenyl)-O-beta-d-glucopyronurona te: a positron emission tomographic tracer for imaging beta-glucuronidase activity in a tumor/inflammation rodent model. Mol. Imaging. 2012;11:77–87. E71. [PubMed] [Google Scholar]
- 5.Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum L.S., Decad G.M., Matthews H.B. Disposition and excretion of 2,3,7,8-tetrachlorodibenzofuran in the rat. Toxicol. Appl. Pharmacol. 1980;55:342–352. doi: 10.1016/0041-008x(80)90096-4. [DOI] [PubMed] [Google Scholar]
- 7.BSEF . Bromine Science and Environment Forum; 2012. TBBPA Factsheet.http://www.bsef.com/uploads/Factsheet_TBBPA_25-10-2012.pdf [Google Scholar]
- 8.Canada . In: Risk Management Scope for Phenol, 4,4′-(1-methylethylidene) bis[2,6-dibromo-(Tetrabromobisphenol A) Chemical Abstract Service Registry Number (CAS RN): 79-94-7. D.o.H. Dept. Of the Environment, editor. 2012. [Google Scholar]
- 9.Carignan C.C., Abdallah M.A., Wu N., Heiger-Bernays W., McClean M.D., Harrad S., Webster T.F. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environ. Sci. Technol. 2012;46:12146–12153. doi: 10.1021/es302638d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cariou R., Antignac J.P., Zalko D., Berrebi A., Cravedi J.P., Maume D., Marchand P., Monteau F., Riu A., Andre F., Le Bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 11.Chignell C.F., Han S.K., Mouithys-Mickalad A., Sik R.H., Stadler K., Kadiiska M.B. EPR studies of in vivo radical production by 3,3′,5,5′-tetrabromobisphenol A (TBBPA) in the Sprague-Dawley rat. Toxicol. Appl. Pharmacol. 2008;230:17–22. doi: 10.1016/j.taap.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Covaci A., Harrad S., Abdallah M.A., Ali N., Law R.J., Herzke D., de Wit C.A. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ. Int. 2011;37:532–556. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 13.de Wit C.A., Herzke D., Vorkamp K. Brominated flame retardants in the Arctic environment – trends and new candidates. Sci. Total Environ. 2010;408:2885–2918. doi: 10.1016/j.scitotenv.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Dixon R.L., Shultice R.W., Fouts J.R. Factors affecting drug metabolism by liver microsomes. IV. Starvation. Proc. Soc. Exp. Biol. Med. 1960;103:333–335. doi: 10.3181/00379727-103-25509. [DOI] [PubMed] [Google Scholar]
- 15.Dotta A., Chukhlantseva N. Ontogeny and drug metabolism in newborns. J. Matern. Fetal Neonatal Med. 2012;25(Suppl. 4):83–84. doi: 10.3109/14767058.2012.715463. [DOI] [PubMed] [Google Scholar]
- 16.Gosavi R.A., Knudsen G.A., Birnbaum L.S., Pedersen L.C. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ. Health Perspect. 2013;121:1194–1199. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagmar L., Sjodin A., Hoglund P., Thuresson K., Rylander L., Bergman A. Biological half-lives of polybrominated diphenyl ethers and tetrabromobisphenol A in exposed workers. Organohalogen Compd. 2000;47:198–201. [Google Scholar]
- 18.Hakk H., Larsen G., Bergman A., Orn U. Metabolism, excretion and distribution of the flame retardant tetrabromobisphenol-A in conventional and bile-duct cannulated rats. Xenobiotica. 2000;30:881–890. doi: 10.1080/004982500433309. [DOI] [PubMed] [Google Scholar]
- 19.Hamers T., Kamstra J.H., Sonneveld E., Murk A.J., Kester M.H., Andersson P.L., Legler J., Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 20.Hamers T., Kamstra J.H., Sonneveld E., Murk A.J., Visser T.J., Van Velzen M.J., Brouwer A., Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol. Nutr. Food Res. 2008;52:284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- 21.IPCS/WHO . In: Tetrabromobisphenol A and Derivatives. World Health Organization G., editor. 1995. [Google Scholar]
- 22.Jakobsson K., Thuresson K., Rylander L., Sjodin A., Hagmar L., Bergman A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 23.Kang M.J., Kim J.H., Lee S.K., Kang W., Kim H.S., Lyoo W.S., Jeong T.C. A rapid method to determine tetrabromobisphenol a in rat serum and urine by liquid chromatography-tandem mass spectrometry. Arch. Pharmacal Res. 2010;33:1797–1803. doi: 10.1007/s12272-010-1112-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang M.J., Kim J.H., Shin S., Choi J.H., Lee S.K., Kim H.S., Kim N.D., Kang G.W., Jeong H.G., Kang W., Chun Y.J., Jeong T.C. Nephrotoxic potential and toxicokinetics of tetrabromobisphenol A in rat for risk assessment. J. Toxicol. Environ. Health A. 2009;72:1439–1445. doi: 10.1080/15287390903212907. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura S., Jinno N., Ohta S., Kuroki H., Fujimoto N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 2002;293:554–559. doi: 10.1016/S0006-291X(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura S., Kato T., Iida M., Jinno N., Suzuki T., Ohta S., Fujimoto N., Hanada H., Kashiwagi K., Kashiwagi A. Anti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: Affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci. 2005;76:1589–1601. doi: 10.1016/j.lfs.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Kuester R.K., Solyom A.M., Rodriguez V.P., Sipes I.G. The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol. Sci. 2007;96:237–245. doi: 10.1093/toxsci/kfm006. [DOI] [PubMed] [Google Scholar]
- 28.Meerts I.A., van Zanden J.J., Luijks E.A., van Leeuwen-Bol I., Marsh G., Jakobsson E., Bergman A., Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell S.J., Hilmer S.N. Drug-induced liver injury in older adults. Ther. Adv. Drug Saf. 2010;1:65–77. doi: 10.1177/2042098610386281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NTP . National Toxicology Program, Health and Human Services; 2013. TR-587. Technical Report Pathology Tables and Curves for TR-587: Tetrabromobisphenol A (TBBPA) Found at: http://ntp.niehs.nih.gov/go/38602. [Google Scholar]
- 31.Riu A., Grimaldi M., le Maire A., Bey G., Phillips K., Boulahtouf A., Perdu E., Zalko D., Bourguet W., Balaguer P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riu A., le Maire A., Grimaldi M., Audebert M., Hillenweck A., Bourguet W., Balaguer P., Zalko D. Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci. 2011;122:372–382. doi: 10.1093/toxsci/kfr132. [DOI] [PubMed] [Google Scholar]
- 33.Samuelsen M., Olsen C., Holme J.A., Meussen-Elholm E., Bergmann A., Hongslo J.K. Estrogen-like properties of brominated analogs of bisphenol A in the MCF-7 human breast cancer cell line. Cell Biol. Toxicol. 2001;17:139–151. doi: 10.1023/a:1011974012602. [DOI] [PubMed] [Google Scholar]
- 34.Schauer U.M., Volkel W., Dekant W. Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol. Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- 35.Shi Z.-X., Wu Y.-N., Li J.-G., Zhao Y.-F., Feng J.-F. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk. Environ. Sci. Technol. 2009;43:4314–4319. doi: 10.1021/es8035626. [DOI] [PubMed] [Google Scholar]
- 36.Shi Z., Jiao Y., Hu Y., Sun Z., Zhou X., Feng J., Li J., Wu Y. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ. 2013;452–453:10–18. doi: 10.1016/j.scitotenv.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Szymanska J.A., Piotrowski J.K., Frydrych B. Hepatotoxicity of tetrabromobisphenol-A: effects of repeated dosage in rats. Toxicology. 2000;142:87–95. doi: 10.1016/s0300-483x(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 38.Szymanska J.A., Sapota A., Frydrych B. The disposition and metabolism of tetrabromobisphenol-A after a single i.p. dose in the rat. Chemosphere. 2001;45:693–700. doi: 10.1016/s0045-6535(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 39.Uhnakova B., Ludwig R., Peknicova J., Homolka L., Lisa L., Sulc M., Petrickova A., Elzeinova F., Pelantova H., Monti D., Kren V., Haltrich D., Martinkova L. Biodegradation of tetrabromobisphenol A by oxidases in basidiomycetous fungi and estrogenic activity of the biotransformation products. Bioresour. Technol. 2011;102:9409–9415. doi: 10.1016/j.biortech.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Van der Ven L.T., Van de Kuil T., Verhoef A., Verwer C.M., Lilienthal H., Leonards P.E., Schauer U.M., Canton R.F., Litens S., De Jong F.H., Visser T.J., Dekant W., Stern N., Hakansson H., Slob W., Van den Berg M., Vos J.G., Piersma A.H. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology. 2008;245:76–89. doi: 10.1016/j.tox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Zalko D., Prouillac C., Riu A., Perdu E., Dolo L., Jouanin I., Canlet C., Debrauwer L., Cravedi J.P. Biotransformation of the flame retardant tetrabromo-bisphenol A by human and rat sub-cellular liver fractions. Chemosphere. 2006;64:318–327. doi: 10.1016/j.chemosphere.2005.12.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.