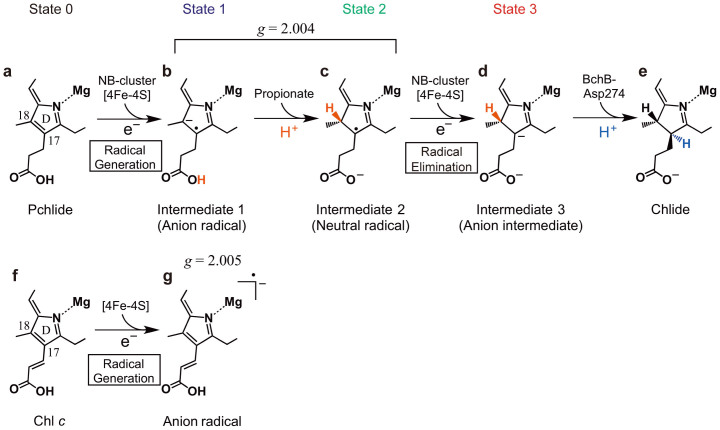
Figure 5.
Proposed reaction steps in the Pchlide reduction (a–e). First step is the electron transfer from the NB-cluster to Pchlide (a) to form a Pchlide anion radical (b) followed by the proton transfer from the propionate to form a neutral radical (c). The second electron transfer eliminates the neutral radical by reduction to form an anion intermediate (d). The Pchlide reduction is completed by the second proton transfer (e). When Chl c is used instead of Pchlide (f), a Chl c anion radical is generated (g).