Figure 5.
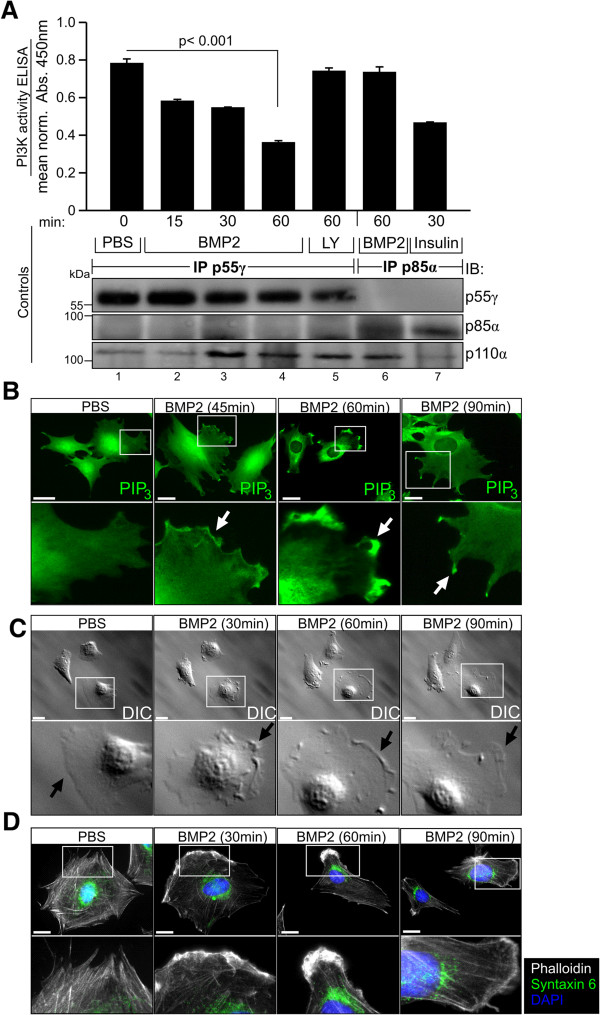
BMP2-induced PIP3 production is p55γ-dependent and localises to cortical actin during lamellipodia formation. (A) BMP2-dependent PIP3 production as detected by PI3K activity ELISA (upper panel). C2C12 cells were stimulated with respective ligands and inhibitors for the indicated times and lysates were subjected to pull-down of endogenous p55γ or p85α as shown. Precipitates were subjected to an in vitro kinase reaction and competitive ELISA was used to detect the amount of PIP3 produced by BMP2-induced PI3K activity. Low absorbance at 450 nm indicates high levels of PIP3. To prove the presence of catalytic p110α in PI3K regulatory subunit pull-down, bead lysates of all three assays were pooled and subjected to detection of p55γ, p85α and p110α protein respectively (lower panel). Error bars represent standard deviation from three independent experiments. (B) Immunocytochemical detection of PIP3 in BMP2 (10 nM) stimulated C2C12 cells by use of PIP3-specific antibody. The cortical region of PIP3 accumulation is indicated by the white arrow. (C) Differential interference contrast (DIC) microscopy of membrane ruffles at dorsal (white arrow) regions of the leading edge of C2C12 cells stimulated with 10 nM BMP2. In B and C, the lower boxes depict magnifications of the regions of interest indicated by white squares (upper boxes). Scale bars represent 20 μm. (D) Phalloidin and Synatxin 6 staining indicating trans-Golgi position facing the cortical actin-rich leading edge of C2C12 cells stimulated with 10 nM BMP2 for the indicated time. Scale bars represent 10 μm. (See also Additional file 5: Figure S5).
