Abstract
The ‘third pole’ of the world is a fitting metaphor for the Himalayan–Tibetan Plateau, in allusion to its vast frozen terrain, rivalling the Arctic and Antarctic, at high altitude but low latitude. Living Tibetan and arctic mammals share adaptations to freezing temperatures such as long and thick winter fur in arctic muskox and Tibetan yak, and for carnivorans, a more predatory niche. Here, we report, to our knowledge, the first evolutionary link between an Early Pliocene (3.60–5.08 Myr ago) fox, Vulpes qiuzhudingi new species, from the Himalaya (Zanda Basin) and Kunlun Mountain (Kunlun Pass Basin) and the modern arctic fox Vulpes lagopus in the polar region. A highly hypercarnivorous dentition of the new fox bears a striking resemblance to that of V. lagopus and substantially predates the previous oldest records of the arctic fox by 3–4 Myr. The low latitude, high-altitude Tibetan Plateau is separated from the nearest modern arctic fox geographical range by at least 2000 km. The apparent connection between an ancestral high-elevation species and its modern polar descendant is consistent with our ‘Out-of-Tibet’ hypothesis postulating that high-altitude Tibet was a training ground for cold-environment adaptations well before the start of the Ice Age.
Keywords: Himalaya, Tibet, arctic fox, Canidae, Pliocene, zoogeography
1. Introduction
Marcel Kurz, the great Swiss geographer, mountaineer and explorer, was credited for coining the term ‘third pole of the globe’ in 1933, referring to the highest point on the Earth, Mount Everest [1]. Owing to their lofty height and bitter cold temperatures, the Himalaya Range, which includes Mount Everest, and the adjacent Tibetan Plateau is endowed with more frozen terrain than anywhere else on the Earth except the North and South poles. Perhaps not surprisingly, living arctic and Tibetan mammals also share similar adaptations to cold climates, such as long, thick winter fur in arctic muskox and Tibetan yak. Previously, we documented the Tibetan origin of the cold-loving woolly rhinoceros and proposed an ‘Out-of-Tibet’ hypothesis, in which the Tibetan Plateau served as a cradle of Ice Age megafauna in northern Eurasia [2]. Furthermore, we also documented a deep-time Tibetan origin of a high-altitude Panthera lineage [3], an early precursor of running hyaena Chasmaporthetes [4], as well as a wolf-sized, hypercarnivorous canid [5]. To these, we now add another example, one which links the modern arctic fauna with historic high Tibet. For the first time to our knowledge, we report a Tibetan link to the arctic fox in our discovery of the oldest fox from the Early Pliocene of the Himalaya and Kunlun ranges. This apparent evolutionary connection between an ancestral high-elevation species and its modern polar descendant offers hints at faunal dynamics that connected two distinct regions of northern Eurasia.
Presence of a fossil relative of arctic fox and other hypercarnivorous species in Pliocene Tibet suggests a carnivoran guild dominated by highly predaceous forms, characteristic of present-day arctic guilds, such as the arctic fox, grey wolf and polar bear. Wintering in extremely cold climates may have played a role in driving such adaptations.
2. Systematic palaeontology
The new fossil fox is described as follows: Carnivora Bowdich, 1821; Canidae Fischer de Waldheim, 1817; Caninae Fischer de Waldheim, 1817; Vulpini Hemprich and Ehrenberg, 1832; Vulpes Frisch, 1775. Vulpes qiuzhudingi sp. nov.
Holotype. Nearly complete left hemimandible with c1, p1 alveolus, p2, p3-4 alveoli, m1 and m2 alveolus; Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Beijing, People's Republic of China, specimen number V18923, collected by an excavation crew led by Gary T. Takeuchi, Zhijie J. Tseng and Qiang Li in 7–14 August 2010.
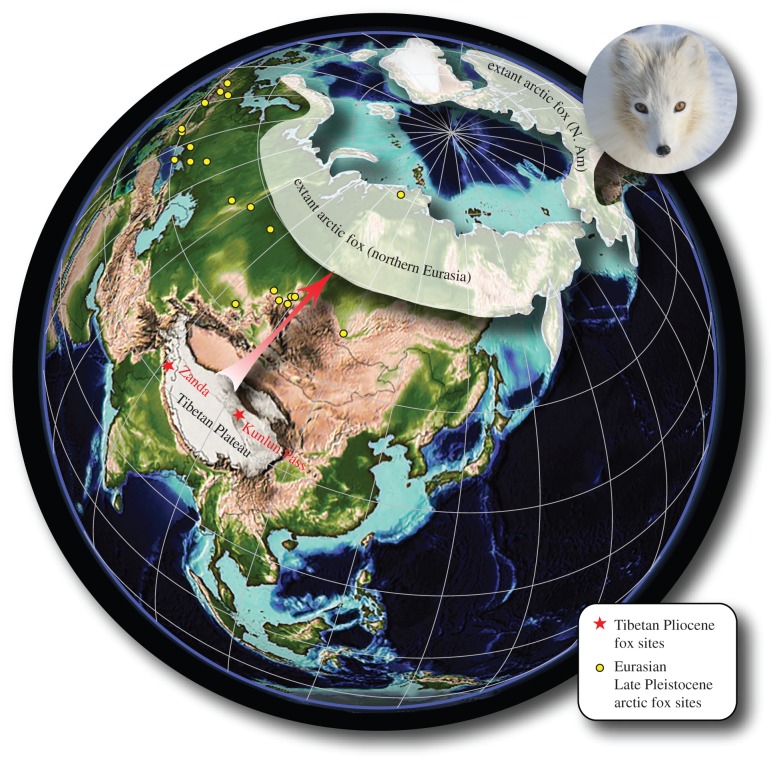
Referred specimen. IVPP V18924, right ramal fragment with broken c1-p4, from IVPP locality ZD1055, 31°30′39″ N, 79°49′04″ E, near the entrance of the Ba'ergou, Zanda Basin in Zanda County, Ali District, Tibetan Autonomous Region, People's Republic of China; collected by Deng Tao on 4 July 2012 (electronic supplementary material, figure S1). IVPP V19060, isolated lower m2 from IVPP locality KL0605, 35°39′13″ N, 94°03′29″ E, Kunlun Pass Basin, Qinghai Province, People's Republic of China; collected by Qiang Li on 25 August 2006 (figure 1).
Figure 1.
Map of Pliocene Tibetan fox localities (stars), Late Pleistocene arctic fox localities (circles), and extant arctic fox (Vulpes lagopus) distribution. Modern distribution is modified from [6], Eurasian Late Pleistocene sites were downloaded from the NOW database on 2 December 2013 [7]. Map of Pliocene palaeogeographic reconstruction is adopted from EarthViewer app. (5 Ma) developed by the Howard Hughes Medical Institute (http://www.hhmi.org//biointeractive/earthviewer). (Online version in colour.)
Type locality. IVPP locality ZD1001, 31°39′58″ N, 79°44′57″ E, elevation 4114 m above sea level (m.a.s.l.), in Zandagou, Zanda Basin, northern foothill of the Himalaya Range, Zanda County, Ali District, Tibetan Autonomous Region, People's Republic of China [8] (figure 1 and the electronic supplementary material, figure S1).
Etymology. In honour of Prof. Qiu Zhuding of the Chinese Academy of Sciences.
3. Diagnosis
About the size of a large male red fox (Vulpes vulpes), V. qiuzhudingi is approximately 20% larger than living and Late Pleistocene arctic foxes. Vulpes qiuzhudingi differs from all other vulpine foxes in having highly hypercarnivorous dentition with trenchant m1–2 talonids dominated by tall hypoconids and a remnant or no entoconid, narrow talonid; m3 is absent.
4. Description
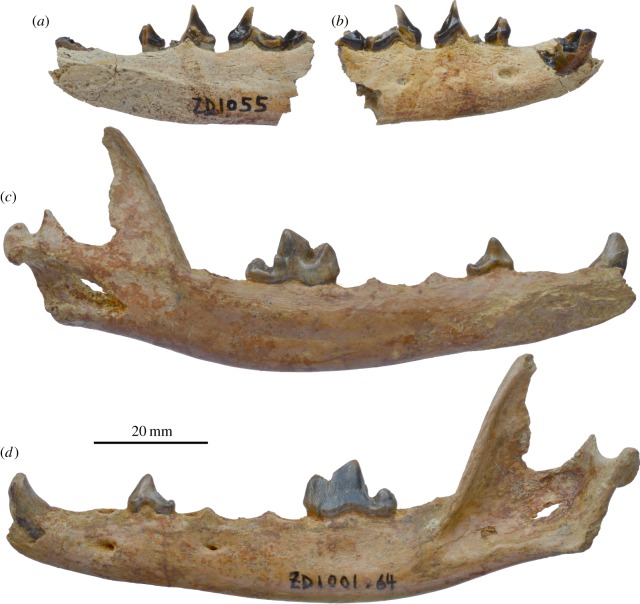
The left hemimandible in the holotype is nearly complete except the ascending ramus. The mandibular corpus is slender and elongated, typical of all Vulpes, with a maximum length from rostral tip to glenoid condyle being 111 mm and maximum depth below the m1 talonid being 15.9 mm. Two mental foramina are present, one directly below the p1 and another at the anterior edge of p3 posterior root. The positions of these two foramina in IVPP V18924 are slightly more forward (figure 2). There is no indication of a subangular process, which is present in the raccoon dog Nyctereutes. The angular process is broken off at base and its morphology cannot be ascertained.
Figure 2.
Vulpes qiuzhudingi new species from Zanda Basin. IVPP V18924, right hemimandible fragment, (a) lingual view and (b) buccal view; IVPP V18923, holotype, left hemimandible, (c) lingual view and (d) buccal view. (Online version in colour.)
The alveolar area for the lower incisors is not well preserved enough to discern the configuration of the i1–3. The c1 is broken in the mid-shaft in the holotype; the remaining basal part indicates a smooth lateral surface and an indistinct cingulum along much of the lingual surface (smoothness along broken enamel edges suggest pre-mortem breakage and the use of the broken canine during life). The p1 is single-rooted and has a single main cusp. The double-rooted p2 has a modest posterior cingular cusp in the holotype, more distinct than many modern foxes, but is less developed in IVPP V18924. Given the limited sample, it is not clear whether these represent ontogenetic (IVPP V18924 being older than IVPP V18923) or individual variations. A broken p3 and p4 is present in IVPP V18924 and indicates a small posterior accessory cusp on p3 and a general lack of an anterior accessory cusp. The m1 is distinctly hypercarnivorous in its slender trigonid with long paraconid–protoconid blade and trenchant talonid. Consistent with this hypercarnivory, there is a much reduced and low-crowned metaconid that is less than 1 mm above the tip of the hypoconid. A distinct vertical groove is present on the posterior face of the trigonid, separating the protoconid from metaconid. The talonid is dominated by a high and large hypoconid that, in occlusal view, makes up almost the entire talonid. A distinct anterior ridge on the hypoconid leads forward to the base of the trigonid, ending in the middle of the posterior trigonid surface. A tiny entoconid is present, attaching at the base of the hypoconid as a posterior extension of a low cingulum. Only the double-rooted m2 alveoli are preserved on the holotype. There is no m3.
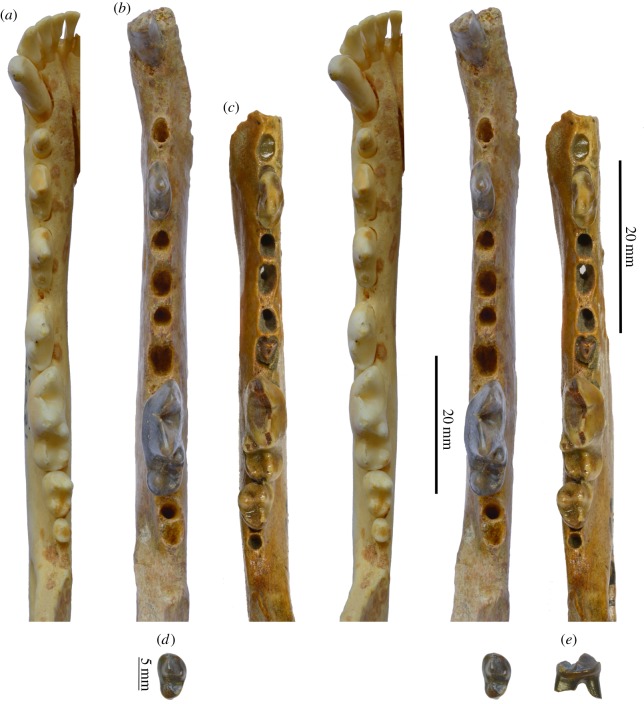
The isolated m2 from IVPP locality KL0605, IVPP V19060, has a highly hypercarnivorous morphology, consistent with the pattern seen in the holotype from Zanda Basin (figure 3d,e). The size of this tooth (length 7.14 mm; width 4.46 mm) matches well with the alveoli in the holotype. The occlusal outline shows a wide trigonid and narrow talonid. A large protoconid completely dominates the much reduced metaconid, which is less than half the height of the protoconid and being displaced to the lingual border of the tooth. The talonid is equally trenchant with a single hypoconid and lack of an entoconid. This m2 represents an even higher altitude (4726 m.a.s.l.) example of this species that is somewhat younger than the Zanda Basin samples and shows slightly more derived morphology indicative of a higher level of hypercarnivory.
Figure 3.
Stereo occlusal views of three fox hemimandibles. (a) Living V. lagopus, left i1-m3, LACM92231 from Alaska; (b) Vulpes qiuzhudingi new species, left c, p2 and m1, IVPP V18923, holotype from Zanda Basin; (c) V. lagopus, right p2, m1-2, F:AM70926 from Rancholabrean of Alaska; (d) stereo occlusal and (e) medial views of Vulpes qiuzhudingi, left m2, IVPP V19060, from IVPP locality KL0605, Kunlun Pass Basin. Scale to the left is for (b), that to the right is for (a,c) and lower one (next to isolated tooth) is for (d,e). (Online version in colour.)
5. Comparison and comment
The Zanda Basin fox is relatively large, near the upper end of the size range in the red fox, V. vulpes, from northern latitudes such as Alaska, Kenai Peninsula, Kodiak Island and Upper Peninsula of Michigan [9,10], and is about 20% larger than arctic foxes based on lower carnassial size [11] (table 1; electronic supplementary material, figure S3). It is smaller than the smallest Canis, e.g. averaging 9% smaller than the golden jackal Canis aureus (based on measurements of five specimens from Iran, India and Ethiopia in the American Museum of Natural History (AMNH) collection), and slightly smaller than Eucyon davisi (mean m1 length = 17.6 mm, range = 16.9–18.0 mm, n = 4) from the Pliocene of Yushe Basin [13]. However, instead of a widened p4 in E. davisi (mean p4 width = 4.8 mm, range = 4.3–5.4 mm, n = 4), that from the Zanda Basin fox is narrowed (4.2 mm; electronic supplementary material, figure S3). It is also much smaller than the Zanda Basin Sinicuon described elsewhere [5]. Interestingly, although the red fox generally follows Bergmann's Rule of increasing body size towards northern latitudes [9], the arctic fox does the opposite, with body size decreasing towards the pole [11,14]. For small carnivorans, McNab [15] argued that body size is mainly a function of prey size, not climate. In this connection, it is also interesting to note that the Zanda Basin V. qiuzhudingi is similar in size to the modern Tibetan sand fox, Vulpes ferrilata, a species that preys largely on pikas and small rodents [16].
Table 1.
Dental measurements of living and fossil arctic foxes. (Measurements for modern European arctic foxes are adopted from [11]. Those for Canadian Arctic (Northwest Territories) are from the AMNH collection, and those for Rancholabrean of Alaska are from the Frick Collection of AMNH, a sample studied by Youngman [12].)
|
Vulpes qiuzhudingi |
Vulpes lagopus |
|||||
|---|---|---|---|---|---|---|
| IVPP V18923 (ZD1001) | IVPP V18924 (ZD1055) | IVPP V19060 (KL0605) | Rancholabrean of Alaska (n = 11) | Europe (n = 122–265) | Northwest Territories, Canada (n = 18) | |
| length of c (mm) | 7.3 | 7.8 | 7.21 | |||
| width of c (mm) | 4.9 | 5.1 | 3.93 | |||
| length of p1 (mm) | 4.9 | 3.93 | 3.81 | 3.71 | ||
| width of p1 (mm) | 2.8 | 2.70 | ||||
| length of p2 (mm) | 8.4 | 8.1 | 7.36 | 7.35 | ||
| width of p2 (mm) | 3.3 | 3.1 | ||||
| length of p3 (mm) | 10.3 | 8.31 | 8.31 | |||
| width of p3 (mm) | 3.3 | |||||
| length of p4 (mm) | 11.7a | 8.94 | 9.09 | |||
| width of p4 (mm) | 4.2 | 3.98 | 4.10 | |||
| length of m1 (mm) | 16.6 | 13.79 | 13.71 | 13.75 | ||
| width of m1 (mm) | 6.1 | 5.15 | 5.13 | 4.96 | ||
| width of m1 talonid (mm) | 5.1 | 5.06 | 4.90 | |||
| length of m2 (mm) | 7.1 | 5.83 | 6.15 | |||
| width of m2 (mm) | 4.5 | 4.09 | 3.94 | |||
aAn estimate based on alveolar length.
With the exception of the arctic fox, all living fox species retain a basined, bicuspid talonid on m1, such as V. vulpes, Vulpes rueppelli, Vulpes chama, Vulpes bengalensis, V. ferrilata, Vulpes velox and Vulpes macrotis. In addition, although being the most variable tooth [10], the m3 is almost always present in red fox and when absent, there are often signs of loss of the tooth during life and reabsorption of the root. Given the above combination of hypercarnivorous characteristics seen only in the arctic fox (figures 2 and 3; electronic supplementary material, figure S2), it seems likely that the new Tibetan fox falls within the arctic fox clade, although possibilities exist that such shared derived characteristics can potentially be due to convergence as seen in multiple lineages of wolf-sized forms during the Pleistocene [17]. Recent molecular phylogeny based on approximately 15 kb of exon and intron sequence places the arctic fox and kit fox as sister species [18], as was also shown in earlier mitochondrial DNA studies [19,20]. However, the kit fox retains the primitively bicuspid talonid, in contrast to the Vulpes lagopus–qiuzhudingi lineage that has a trenchant m1 talonid.
A dolichocephalic skull, in which the rostrum is elongated, is one of the most salient features of the Tibetan sand fox, V. ferrilata, in addition to having relatively short legs [16,21]. This rostral elongation is also reflected in the wide spacing (long diastemata) between upper premolars. Although Zanda Basin fox fossils generally fall in the size range of V. ferrilata, its highly hypercarnivorous m1–2 with enlarged hypoconid at the expense of entoconid, as well as associated dental features such as reduced metaconid and loss of m3, are very different from that of V. ferrilata. Given that a hypercarnivorous dentition generally does not reverse back to more primitive, mesocarnivorous conditions in the evolutionary history of canids [17,22,23], and the general trend that hypercarnivory is also frequently associated with body size increase [24], we think it highly unlikely that the new Zanda Basin fox could be related to the modern Tibetan sand fox. However, as shown below, the Tibetan sand fox itself may be linked to the arctic fox.
Until recently, phylogenetic work on the Tibetan sand fox was unavailable and the only published assessment was based on a numerical classification of phenotypic characteristics, in which V. ferrilata was closest to the corsac fox (steppe fox) [25], as was also reflected, not surprisingly, in a supertree analysis of crown Carnivora [26]. However, a strict consensus of 12 trees by Zrzavý & Řičánková [27] from a combined dataset of morphology, cytochrome b and cytochrome c oxidase subunits I and II yielded no resolution (nearly all species of Vulpes fall within a multichotomy). Most intriguingly a recent total evidence phylogeny of living Caninae, based on a data matrix of morphologic, molecular, cytogenetic, life history, ecologic and behavioural characteristics, fully resolved the Vulpes relationship and placed the artic fox (V. lagopus) and Tibetan sand fox (V. ferrilata) at the base of a four-species clade along with the kit fox (V. macrotis) and swift fox (V. velox) at the terminal end [28]. This is the first time, to our knowledge, the Tibetan sand fox has been linked to the arctic fox in a phylogeny, although presently described fossil species from the Pliocene of Tibet was presumably already within the arctic fox species complex.
6. Zoogeography and palaeoenvironment
Several morphological and physiological adaptations enable the survival of the arctic fox in harsh, bitterly cold arctic conditions: long, thick winter fur with 70% fine underfur, compact body, short ears and legs, foot thermoregulation (capillary rete in the skin of the pads) and reduced metabolism during cold weather [6,29]. Interestingly, the Tibetan sand fox, V. ferrilata, seems to be a close parallel in these adaptations (such as a thick fur) [16], which may have been acquired in their common ancestors in Tibet (see above). This implies that two closely related lineages of foxes, V. qiuzhudingi–lagopus lineage and V. ferrilata, have evolved in the Tibetan Plateau that are adapted to cold climates, one evolving hypercarnivory and giving rise to the arctic fox lineage and another remaining primitive in its dental morphology and giving rise to the Tibetan sand fox.
Stable isotope analyses suggest that the Pliocene Zanda Basin was close to [30] or a few degrees warmer than [31] the present-day temperatures of 0°C annual average. During the winters, temperatures were likely to be quite cold. By contrast, the arctic Pliocene is as much as 8°C or more warmer than it is today [32–34]. This points to the interesting possibility that Pliocene high Tibet was a harsher environment than its arctic counterparts, thus presenting a more severe environmental challenge to its foxes.
With the discovery of our new Tibetan form, we now trace the origin of the arctic fox clade to the high-elevation Tibetan Plateau at a much earlier time than previously thought but having very characteristic and consistent hypercarnivorous morphology. This new Tibetan species thus suggests a long hidden record of arctic foxes between their Tibetan first appearance and the much later record of V. lagopus in Europe and North America. However with only three specimens in our collection and given the generally poor record of fossil carnivorans, additional collecting is likely to extend the range to younger strata. How much younger this extension will be is not knowable at the moment, but a general scenario seems warranted: an early appearance of a cold-adapted clade that persisted in the high plateau for several million years before descending and spreading northwards, presumably during the Ice Age, as did the woolly rhinoceros [2].
Discovery of two hypercarnivorous canids in the Pliocene of high Tibet, well before their records appear elsewhere in northern Eurasia, may have ecologic and environmental implications. Modern terrestrial carnivorans in arctic regions, such as the arctic fox, grey wolf and polar bear, subsist almost exclusively on a carnivorous diet [6,35,36]. Such a highly predatory lifestyle seems likely to be a consequence of the availability of food resources, especially during the cold winters and high energetic requirements in freezing temperatures. It is thus of interest to speculate that the predominance of predatory canids in the Pliocene of Tibet may also be related to surviving cold winters. The fact that the Tibetan forms predate records elsewhere lends further support for our ‘Out-of-Tibet’ hypothesis [2], in that, more primitive forms of some mammals evolved first in high Tibet at a time when high arctic regions were much warmer, and subsequently gave rise to Ice Age descendants in northern Eurasia.
Supplementary Material
Acknowledgements
We thank members of our field teams in various field seasons—their outstanding contributions, ever cheerful often under difficult circumstances at high elevation, are invaluable in the establishment of a biostratigraphic framework. Access to living mammal collections were made possible by Jim Dines of LACM. The senior author has benefited greatly from numerous discussions with the late Richard H. Tedford on vulpine relationships, as well as from unpublished manuscripts by the latter. We greatly appreciate the digital photography of two foxes in the MCZ collection by Judy Chupasko and Catherine Weisel. We thank Paúl Velazco (AMNH) for bringing to our attention important literature. Critical reviews by Blaire Van Valkenburgh (B.V.V.), Manuel Salesa and Lars Werdelin (L.W.) helped to sharpen several important points. In particular, we appreciate the detailed edits by B.V.V., which greatly improved clarity, and important points about Vulpes systematics raised by L.W.
Data accessibility
All vertebrate fossils from the Tibetan Plateau are housed in the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, in Beijing.
Funding statement
Financial support is provided by the Strategic Priority Research Program of the Chinese Academy of Science (XDB03020104), Major Basic Research Projects of the Ministry of Science and Technology of China (973 Program, No. 2012CB21904), CAS/SAFEA International Partnership Program for Creative Research Teams, Chinese National Natural Science Foundation (no. 40702004 to Q.L., 40730210), Chinese Academy of Science Outstanding Overseas Scholar Fund (KL205208), National Science Foundation (US) (EAR-0446699, 0444073, 0958704, 1227212 to X.W.) and the National Geographic Society (no. W22–08 to Q.L.), and a Frick Postdoctoral Fellowship to Z.J.T.
References
- 1.Dyhrenfurth GO. 1955. To the third pole: the history of the high Himalaya. London, UK: Werner Laurie. [Google Scholar]
- 2.Deng T, et al. 2011. Out of Tibet: Pliocene woolly rhino suggests high-plateau origin of Ice Age megaherbivores. Science 333, 1285–1288. ( 10.1126/science.1206594) [DOI] [PubMed] [Google Scholar]
- 3.Tseng ZJ, Wang X, Slater GJ, Takeuchi GT, Li Q, Liu J, Xie G. 2013. Himalayan fossils of the oldest known pantherine establish ancient origin of big cats. Proc. R. Soc. B 281, 20132686 ( 10.1098/rspb.2013.2686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng ZJ, Li Q, Wang X. 2013. A new cursorial hyena from Tibet, and analysis of biostratigraphy, paleozoogeography, and dental morphology of Chasmaporthetes (Mammalia, Carnivora). J. Vert. Paleontol. 33, 1457–1471. ( 10.1080/02724634.2013.775142) [DOI] [Google Scholar]
- 5.Wang X, Li Q, Xie G. In press. Earliest record of Sinicuon in Zanda Basin, southern Tibet and implications for hypercarnivores in cold environments. Quat. Int. ( 10.1016/j.quaint.2014.03.028) [DOI] [Google Scholar]
- 6.Audet AM, Robbins CB, Larivière S. 2002. Alopex lagopus Mamm. Species 713, 1–10. () [DOI] [Google Scholar]
- 7.Fortelius M. 2013. New and Old Worlds database of fossil mammals (NOW), University of Helsinki See http://www.helsinki.fi/science/now/.
- 8.Wang X, et al. 2013. Mio-Pleistocene Zanda Basin biostratigraphy and geochronology, pre-Ice Age fauna, and mammalian evolution in western Himalaya. Palaeogeogr. Palaeoclim. Palaeoecol. 374, 81–95. ( 10.1016/j.palaeo.2013.01.007) [DOI] [Google Scholar]
- 9.Szuma E. 2008. Evolutionary and climatic factors affecting tooth size in the red fox Vulpes vulpes in the Holarctic. Acta Theriol. 53, 289–332. ( 10.1007/BF03195193) [DOI] [Google Scholar]
- 10.Gingerich PD, Winkler DA. 1979. Patterns of variation and correlation in the dentition of the red fox, Vulpes vuples. J. Mammal. 60, 691–704. ( 10.2307/1380186) [DOI] [Google Scholar]
- 11.Szuma E. 2008. Geographic variation of tooth and skull sizes in the arctic fox Vulpes (Alopex) lagopus. Ann. Zool. Fennici 45, 185–199. ( 10.5735/086.045.0304) [DOI] [Google Scholar]
- 12.Youngman P. 1993. The Pleistocene small carnivores of eastern Beringia. Can. Field Nat. 107, 139–163. [Google Scholar]
- 13.Tedford RH, Qiu Z-X. 1996. A new canid genus from the Pliocene of Yushe, Shanxi Province. Vert. PalAsiat 34, 27–40. [Google Scholar]
- 14.Germonpre M, Sablin MV. 2004. Systematics and osteometry of Late Glacial foxes from Belgium. Bull. Inst. R. Sci. Nat. Beig. Sci. Terre 74, 175–188. [Google Scholar]
- 15.McNab BK. 1971. On the ecological significance of Bergmann's Rule. Ecology 52, 845–854. ( 10.2307/1936032) [DOI] [Google Scholar]
- 16.Clark HO, Newman DP, Murdoch JD, Tseng J, Wang ZH, Harris RB. 2008. Vulpes ferrilata Mamm. Species 821, 1–6. ( 10.1644/821.1) [DOI] [Google Scholar]
- 17.Tedford RH, Wang X, Taylor BE. 2009. Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae). Bull. Am. Mus. Nat. Hist. 325, 1–218. ( 10.1206/574.1) [DOI] [Google Scholar]
- 18.Lindblad-Toh K, et al. 2005. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819. ( 10.1038/nature04338) [DOI] [PubMed] [Google Scholar]
- 19.Mercure A, Ralls K, Koepfli KP, Wayne RK. 1993. Genetic subdivisions among small canids: mitochondrial DNA differentiation of swift, kit, and arctic foxes. Evolution 47, 1313–1328. ( 10.2307/2410150) [DOI] [PubMed] [Google Scholar]
- 20.Geffen E, Mercure A, Girman DJ, Macdonald DW, Wayne RK. 1992. Phylogenetic relationships of the fox-like canids: mitochondrial DNA restriction fragment, site and cytochrome b sequence analyses. J. Zool. 228, 27–39. ( 10.1111/j.1469-7998.1992.tb04430.x) [DOI] [Google Scholar]
- 21.Pocock RI. 1937. The foxes of British India. J. Bombay Nat. Hist. Soc. 39, 36–57. [Google Scholar]
- 22.Wang X, Tedford RH, Taylor BE. 1999. Phylogenetic systematics of the Borophaginae (Carnivora: Canidae). Bull. Am. Mus. Nat. Hist. 243, 1–391. [Google Scholar]
- 23.Wang X. 1994. Phylogenetic systematics of the Hesperocyoninae (Carnivora: Canidae). Bull. Am. Mus. Nat. Hist. 221, 1–207. [Google Scholar]
- 24.Van Valkenburgh B, Wang X, Damuth J. 2004. Cope's rule, hypercarnivory, and extinction in North American canids. Science 306, 101–104. ( 10.1126/science.1102417) [DOI] [PubMed] [Google Scholar]
- 25.Clutton-Brock J, Corbet GB, Hills M. 1976. A review of the family Canidae, with a classification by numerical methods. Bull. Br. Mus. Nat. Hist. Zool. 29, 117–199. [Google Scholar]
- 26.Bininda-Emonds ORP, Gittleman JL, Purvis A. 1999. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biol. Rev. 74, 143–175. ( 10.1017/S0006323199005307) [DOI] [PubMed] [Google Scholar]
- 27.Zrzavý J, Řičánková V. 2004. Phylogeny of recent Canidae (Mammalia, Carnivora): relative reliability and utility of morphological and molecular datasets. Zool. Scripta 33, 311–333. ( 10.1111/j.0300-3256.2004.00152.x) [DOI] [Google Scholar]
- 28.Fuentes-González JA, Muñoz-Durán J. 2012. Filogenia de los cánidos actuales (Carnivora: Canidae) mediante análisis de congruencia de characteres bajo parsimonia. Actual Biol. 34, 85–102. [Google Scholar]
- 29.Prestrud P. 1991. Adaptations by the arctic fox (Alopex lagopus) to the polar winter. Arctic 44, 132–138. ( 10.2307/40511073) [DOI] [Google Scholar]
- 30.Saylor JE, Quade J, Dettman DL, DeCelles PG, Kapp PA, Ding L. 2009. The Late Miocene through present paleoelevation history of southwestern Tibet. Am. J. Sci. 309, 1–42. ( 10.2475/01.2009.01) [DOI] [Google Scholar]
- 31.Wang Y, et al. 2013. Diet and environment of a mid-Pliocene fauna from southwestern Himalaya: paleo-elevation implications. Earth Planet. Sci. Lett. 376, 43–53. ( 10.1016/j.epsl.2013.06.014) [DOI] [Google Scholar]
- 32.Brigham-Grette J, et al. 2013. Pliocene warmth, polar amplification, and stepped Pleistocene cooling recorded in NE Arctic Russia. Science 340, 1421–1427. ( 10.1126/science.1233137) [DOI] [PubMed] [Google Scholar]
- 33.Ballantyne AP, Greenwood DR, Sinninghe Damsté JS, Csank AZ, Eberle JJ, Rybczynski N. 2010. Significantly warmer Arctic surface temperatures during the Pliocene indicated by multiple independent proxies. Geology 38, 603–606. ( 10.1130/g30815.1) [DOI] [Google Scholar]
- 34.Csank AZ, Tripati AK, Patterson WP, Eagle RA, Rybczynski N, Ballantyne AP, Eiler JM. 2011. Estimates of Arctic land surface temperatures during the early Pliocene from two novel proxies. Earth Planet. Sci. Lett. 304, 291–299. ( 10.1016/j.epsl.2011.02.030) [DOI] [Google Scholar]
- 35.DeMaster DP, Stirling I. 1981. Ursus maritimus Mamm. Species 145, 1–7. ( 10.2307/3503828) [DOI] [Google Scholar]
- 36.Mech LD. 1974. Canis lupus Mamm. Species 37, 1–6. ( 10.2307/3503924) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All vertebrate fossils from the Tibetan Plateau are housed in the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, in Beijing.