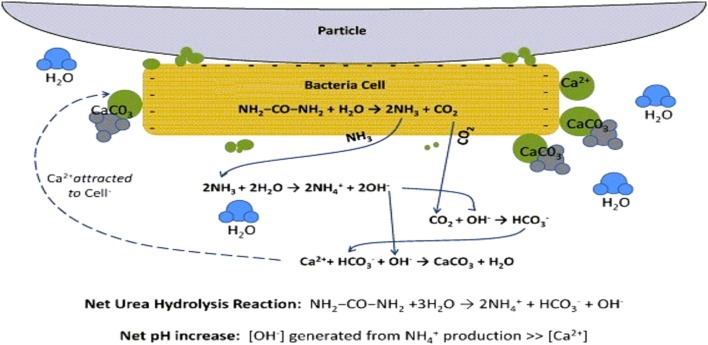
Figure 1.
Bacteria serving as nucleation site for CaCO3 precipitation in the substrate particles. Calcium ions in the solution are attracted to the bacterial cell wall due to its negative charge. When urea is added to bacteria, dissolved inorganic carbon (DIC) and ammonium (AMM) are released in the microenvironment of the bacteria. In the presence of calcium ions, this leads to local supersaturation and finally there is precipitation of calcium carbonates which act as binder between loose substrate particles (Source: DeJong et al., 2010).