Abstract
Objective:
This study was aimed to investigate the beneficial effects of quercetin (QCT) against trinitrobenzene sulfonic acid (TNBS) induced clinical, morphological, and biochemical alterations in rats.
Materials and Methods:
Colitis in rats was induced by administration of TNBS (25 mg dissolved in 0.25 ml of 30% ethanol) 8 cm into the rectum of the rat using a catheter. The animals were divided into six experimental groups (n = 6); naive (saline only without TNBS administration), control (saline + TNBS), standard (sulfasalazine 25 mg/kg + TNBS), QCT (25) (QCT 25 mg/kg + TNBS), QCT (50) (QCT 50 mg/kg + TNBS), QCT (100) (QCT 100 mg/kg + TNBS). Sulfasalazine (25 mg/kg) and QCT (25, 50 and 100 mg/kg) were administered per oral for 11 days and the colonic damage was evaluated in terms of macroscopical (body weight, stool consistency, rectal bleeding, and ulcer index) and biochemical parameters (myeloperoxidase activity, lipid peroxidation, nitrite, and glutathione).
Results:
Treatment with QCT (50, 100 mg/kg) for 10 days following TNBS administration significantly attenuated the clinical, morphological, and biochemical alterations induced by TNBS, whereas it was found to be not effective at its lower dose (25 mg/kg) throughout the experimental protocol.
Conclusion:
QCT attenuates the clinical, morphological and biochemical alterations induced by TNBS possibly via its antioxidant mechanism.
KEY WORDS: Inflammatory bowel disease, myeloperoxidase, oxidative stress, ulcer index
Introduction
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder characterized by intestinal inflammation, mucosal tissue damage and infiltration of polymorphonuclear leukocytes, monocytes and macrophages. IBD is clinically characterized by two overlapping phenotypes namely ulcerative colitis (UC) and Crohn's disease predominantly affecting the colon and occasionally extending to the distal small intestine in either a superficial or transmural manner.[1,2] UC is a condition in which the inflammatory response and morphologic changes remain confined to the colon and consists of variable severity of ulceration, edema, and hemorrhage along the length of the colon. IBD is generally initiated and perpetuated by a deregulated immune response along with the imbalance between the production of free radical and antioxidant defense in the body.[3,4] A favorable correlation between the activity of free radicals in the intestine and the clinical disease activity has been exhibited indicating the significance of these free radicals in the inflammatory process. Besides, the effectiveness of various therapies in IBD has been suggested to be related to oxidant-antioxidant imbalance.[5]
Colitis can be induced in rats by administration of trinitrobenzene sulfonic acid (TNBS) along with ethanol which disrupts the mucosal layer, leading to the formation of granuloma with infiltration of inflammatory cells in all layers producing large amounts of interleukin (IL)-12, interferon-gamma and IL-2 which is similar to the changes found in human disease. The TNBS model served in various investigations for the development and testing of therapeutic molecules that have the potential to be used for the management of IBD in humans.[6]
Quercetin (QCT) (3,3’,4’,5-7-pentahydroxyflavone), a flavanol chemically related to Kaempferol, prevents oxidative injury and cell death by various mechanisms including oxygen radical scavenging activity, inhibiting xanthene oxidase, lipid peroxidation and chelating metal ions. Besides, QCT has been found to elicit beneficial effects in diabetic, depressant, and hypertensive conditions. Further, QCT has been reported to inhibit the antigen-immunoglobulin E mediated tumor necrosis factors-α and IL-4 production in Type I allergic reactions and also decreases the expression of Th2-type cytokines (IL-4, IL-13, and IL-5) by basophils.[7] Thus, the present work has been undertaken to evaluate the possible protective effect of QCT against TNBS induced IBD like symptoms in rats.
Materials and Methods
Drugs and Chemicals
Sulfasalazine was obtained as a gift sample from Symed Laboratories, Hyderabad, India. TNBS, QCT, hexadecyl trimethyl ammonium bromide (dodecyl trimethylammonium bromide [DTAB]), O-dianisidine and other chemicals were purchased from Sigma Chemicals (St. Louis, MO, USA). Ethanol and other reagents were purchased from Finar reagents, Ahmadabad, India.
Animals
Male Wistar rats (220-250 g) were obtained from Mahaveer Enterprises, Hyderabad, India. The rats were housed at a temperature of 25 ± 1°C and relative humidity of 45-55% under 12:12 light-dark cycle. The animals had free access to feed pellets and water ad libitum. The experimental protocol was approved (Approval No. Committee for Control and Supervision of Experimentation on Animals [CPCSEA] - 2011/10/06/02 dated 17/12/2008) by the Institutional Animal Ethics Committee and performed in accordance with the guidelines of CPCSEA, Government of India on animal experimentation.
Trinitrobenzene Sulphonic Acid Induced Colitis
The rats were divided into six groups (n = 6; each) and were deprived of food for 48 h before the experiment.
Group I: Naive (saline)
Group II: control (saline + TNBS)
Group III: Sulphasalazine (sulphasalazine 25 mg/kg, per oral [p.o.] + TNBS)
Group IV: QCT (25) (QCT 25 mg/kg, p.o. + TNBS)
Group V: QCT (50) (QCT 50 mg/kg, p.o. + TNBS)
Group VI: QCT (100) (QCT 100 mg/kg, p.o. + TNBS).
On the day zero, each rat in Groups II, III, IV and V were anesthetized using anesthetic ether and a single dose (0.25 ml) TNBS was administered 8 cm into the rectum of the rat using catheter at a dose of 25 mg dissolved in 0.25 ml of 30% ethanol. The animals were maintained in the vertical position for 30 s and then returned to their respective cages. Immediately, after TNBS administration, QCT and sulfasalazine were administered p.o. daily in the morning 10: 00 a.m., for 11 days. Body weight, stool consistency and rectal bleeding of each animal were observed daily for 11 days. All the animals were sacrificed by decapitation on the 11th day, colon was isolated and cut open to expose inner surface and washed thoroughly with normal saline and scanned for the morphological analysis.[8]
Body Weight, Stool Consistency and Rectal Bleeding
The body weight of all the animals were measured daily from day 2 till day 11. The stool consistency of all the animals was quantified on a 0-4 scale as 0 points for well-formed pellets, 2 points for pasty and semi-solid stool that did not stick to the anus and 4 points for liquid stools that sticks to the anus, daily from day 1 to day 11. Likewise, in case of rectal bleeding, Score “0” was assigned for no blood, “2” for positive finding, and “4” for gross bleeding.[9]
Ulcer Index
For analysis of ulcer index, the scanned photographs of the colons were analyzed using image J v 1.47 software (U. S. National Institutes of Health, Bethesda, Maryland, USA) and ulcer area was calculated. The ulcer index was obtained by using the following formula.[10]
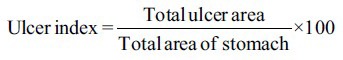
Macroscopic Score
The macroscopic scoring of colon was done on the basis of its macroscopic appearance. Scoring was done by an independent observer according to the scale ranging from 0 to 4.[11] Score 0; no macroscopic changes, Score 1; mucosal erythema only, Score 2; mild mucosal edema with slight bleeding or small erosions, Score 3; moderate edema with bleeding ulcers or erosions and Score 4; severe ulceration, erosions, edema and tissue necrosis.
Myeloperoxidase Activity
Myeloperoxidase activity was determined by a method as previously described by Krawisz et al.[12] Briefly, 100 mg of colon mucosal scrapings were homogenized in one solution containing 0.5% DTAB dissolved in 50 mM potassium phosphate buffer (pH 6) and sonicated in ice bath for 10 s. The homogenates were freeze-thawed thrice and centrifuged for 15 min at 20,000× g. The level of myeloperoxidase (MPO) activity was measured spectrophotometrically: 0.1 ml of the material to be measured was mixed with 2.9 ml of 50 mM phosphate buffer, containing O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was measured for 5 min using a Beckman spectrophotometer (Beckman DU 640B). MPO activity was measured using the following formula:
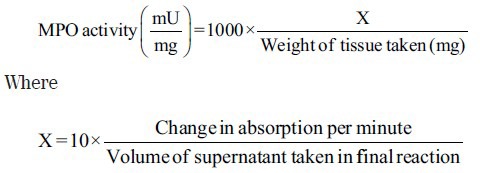
Total Protein
The estimation of protein was done according to a method as described by Lowry et al.[13] Briefly, 0.1 ml of the tissue homogenate was added with 0.8 ml of 0.1 M sodium hydroxide and 5 ml of Lowry C reagent and the solution mixture was allowed to stand for 15 min. Protein concentration was estimated by measuring the absorbance of the solution at 640 nm after adding 0.5 ml of 1N Folin's phenol reagent to the above solution. Protein content was calculated by using bovine serum albumin as standard. The values were expressed as milligram of protein per gram of wet tissue (mg/g).
Determination of Colonic Glutathione Content
Reduced glutathione (GSH) was determined by a method as previously described by Moron et al.[14] Briefly, equal volumes of tissue homogenate and 20% ice cold trichloroacetic acid (TCA) were mixed. The precipitated fraction was centrifuged and to 0.25 ml of supernatant, 2 ml of DTNB reagent was added. The final volume was made up to 3 ml with phosphate buffer. The color developed was read at 412 nm against reagent blank. The amount of reduced GSH was expressed as μg of GSH/mg protein.
Determination of Lipid Peroxidation
Lipid peroxidation was estimated as described by Ohkawa et al.[15] Briefly, 2.0 ml of the tissue homogenate was added to 2.0 ml of freshly prepared 10% w/v TCA and the mixture was allowed to stand in an ice bath for 15 min. After centrifugation, 2.0 ml of clear supernatant solution was mixed with 2.0 ml of freshly prepared thiobarbituric acid. The resulting solution was heated in a boiling water bath for 10 min and immediately cooled in an ice bath for 5 min. The color developed was measured at 532 nm against reagent blank. Malondialdehyde (MDA) concentration was determined using a standard graph. The values were expressed as nmol of MDA/mg protein.
Determination of Serum Nitrite/Nitrate Levels
Serum nitrate levels were estimated according to the method describes by Miranda et al.[16] The absorbance of the azo product formed by the reaction between nitrite, sulfonamide and N-(1-napthyl) ethylenediamine was measured at 543 nm. The concentrations were determined using a standard curve of sodium nitrate and the results were expressed as μmol/l.
Statistical Analysis
The data were analyzed by using one-way analysis of variance (ANOVA) followed by Dunnet's test and two-way ANOVA (body weight, stool consistency, and rectal bleeding) followed by Bonferroni's test. All statistical analyzes were carried out with the help of Graphpad prism version 5 (trial version) software (GraphPad Software, Inc. 7825, Fay Avenue, Suite 230, La Jolla, CA 92037, USA). All the values were expressed as mean ± standard error of the mean and the criterion for statistical significance was considered to be P < 0.05.
Results
Effect of Quercetin on Trinitrobenzene Sulphonic Acid Induced Colonal Damage
Intra rectal administration of TNBS (25 mg) resulted in the development of colitis, characterized by patchy inflammatory lesions and colonic inflammation, while the rats receiving saline (naive) remained free of from these changes. Administration of QCT (50 or 100 mg/kg) and sulfasalazine (25 mg/kg) treatment for 10 days showed noticeable protection against the colonal damage whereas QCT (25 mg/kg) was found to be nonsignificant [Figure 1].
Figure 1.
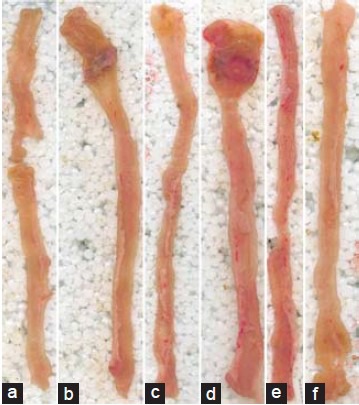
Morphological representation of ulcer formation on colon in trinitrobenzene sulfonic acid (TNBS) induced inflammatory bowel disease (IBD) model. (a) Naive, (b) TNBS control, (c) Sulfasalazine, (d) Quercetin (QCT) (25 mg/kg), (e) QCT (50 mg/kg), (f) QCT (100 mg/kg)
Effect of Quercetin on Body Weight, Stool Consistency and Rectal Bleeding
The body weight of all the animals in naive group was decreased until day 0 possibly due to starvation and after which the body weight has been recovered quickly, whereas the body weights of all the animals in control group were significantly (P < 0.05) decreased until day 11 as compared to naive group. Further, the body weight of all the animals in sulphasalazine or QCT treated groups were decreased till day 3 and thereafter increased significantly (P < 0.05) as compared to control group [Figure 2a]. Likewise, the stool consistency score in control group has been found to be significantly (P < 0.05) higher when compared to the naive group throughout the study [Figure 2b]. Nevertheless, significant rectal bleeding was observed in control group from 1 to 11 days after TNBS administration as compared to naive group, whereas sulfasalazine and QCT treatment showed a significant (P < 0.05) decrease in rectal bleeding from day 3, 5 and 7 respectively in TNBS treated animals when compared with that of the control group [Figure 2c]. Low dose of QCT (25 mg/kg) administration was found to have nonsignificant effect in all these parameters.
Figure 2.
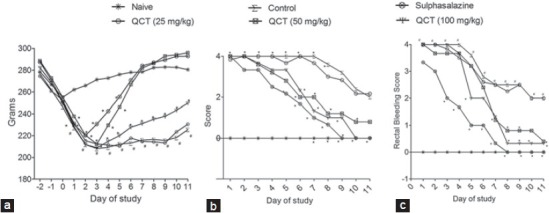
Effect of quercetin on body weight (a) (Dfn5, 13, Dfd 420; F = 357.73, 91.80), Stool consistency, (b) (Dfn5, 13, Dfd 420; F = 307.73, 87.09), Rectal bleeding, (c) (Dfn5, 13, Dfd420; F = 192.33, 65.85) in trinitrobenzene sulphonic acid-induced inflammatory bowel disease like symptoms in rats. Data was analyzed by two-way analysis of variance followed by Bonferroni test. #P < 0.05 as compared to naive group, *P < 0.05, as compared to control group
Effect of Quercetin on Colon Macroscopy and Ulcer Index
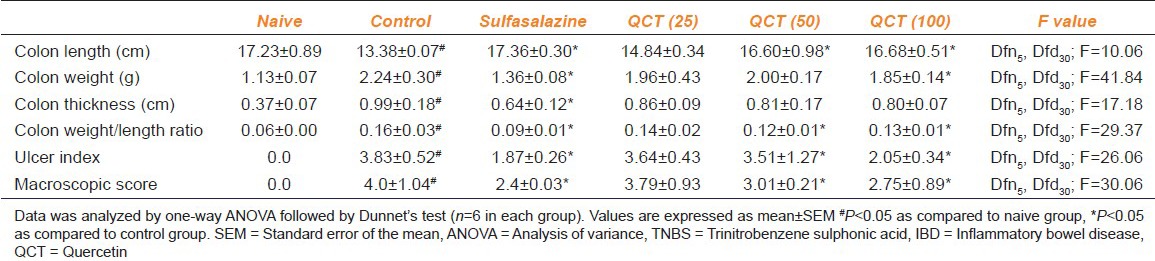
Intra-rectal administration of TNBS in rats induced severe macroscopic inflammation in the colon and it is evident by the macroscopic score, weight of the colon, colon length and colon weight versus length ratio when compared with the naive animals. However, treatment with QCT (50 and 100 mg/kg) or sulphasalazine (25 mg/kg) for 10 days significantly (P < 0.05) ameliorated the alteration in the colon morphology as compared to control group, whereas QCT (25 mg/kg) was found to be nonsignificant [Table 1].
Table 1.
Effect of quercetin on morphological alterations in TNBS induced IBD like symptoms in rats
Effect of Quercetin on Myeloperoxidase Activity
Intra-rectal instillation of TNBS in rats resulted in significant (P < 0.05) increase in MPO activity in control group as compared to naive animals. However, administration of sulphasalazine (25 mg/kg) or QCT (50 and 100 mg/kg) for 11 days ameliorated the increase in MPO activity significantly (P < 0.05) as compared to the control group, whereas QCT (25 mg/kg) was found to be nonsignificant [Figure 3a].
Figure 3.
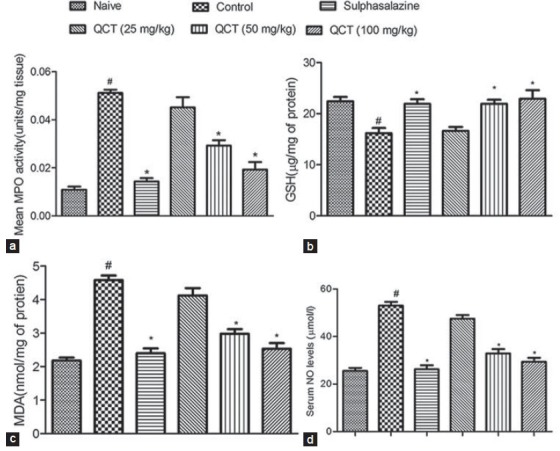
Effect of quercetin on biochemical alterations in trinitrobenzene sulfonic acid induced inflammatory bowel disease like symptoms in rats (a) myeloperoxidase (Dfn5, Dfd30; F = 21.06), (b) glutathione (Dfn5, Dfd30; F = 11.53), (c) malondialdehyde (Dfn5, Dfd30; F = 19.07) and (d) NO levels (Dfn5, Dfd30; F = 20.6). Data were analyzed by one-way analysis of variance followed by Dunnet's test. #P < 0.05 as compared to naive group, *P < 0.05, as compared to control group
Effect of Quercetin on Colonic Glutathione Content
Intra-rectal instillation of TNBS in rats caused a significant (P < 0.05) decreased in GSH levels in control group as compared to naive animals. However, pretreatment with sulfasalazine (25 mg/kg) or QCT at different doses (50 and 100 mg/kg) caused a significant (P < 0.05) increase in the GSH levels as compared to control group, whereas QCT (25 mg/kg) was found to be nonsignificant [Figure 3b].
Effect of Quercetin on Lipid Peroxidation
Intra-rectal instillation of TNBS in rats caused a significant (P < 0.05) increase in the colonic MDA concentration in control group as compared to the naive animals. Treatment with sulfasalazine (25 mg/kg) or QCT (50 and 100 mg/kg) for 11 days offered a significant (P < 0.05) protection to the animals from the TNBS induced increase in MDA levels, whereas QCT (25 mg/kg) was found to be nonsignificant [Figure 3c].
Effect of Quercetin on Serum Nitrite/Nitrate Levels
Intra-rectal instillation of TNBS in rats caused a significant increase (P < 0.05) in nitrite/nitrate levels in control group as compared to naive animals. Administration of sulfasalazine (25 mg/kg) or QCT (50 and 100 mg/kg) treatment produced significant (P < 0.05) decrease in the nitrite/nitrate levels as compared to control group, whereas QCT (25 mg/kg) was found to be nonsignificant [Figure 3d].
Discussion
Inflammatory bowel disease is characterized by diffuse mucosal inflammation limited to the colon. The inflammatory process along with the oxidative injury which results from the activation of cyclooxygenase and lipooxygenase pathways and excessive production of reactive oxygen species (ROS) respectively were considered to be the key factors in the progression of the disease. The symptoms of IBD are more heterogeneous, but typically include abnormality in weight loss, stool consistency and rectal bleeding. However increasing amounts of evidences suggest that intra-rectal instillation of TNBS mimics the IBD like conditions[17] in animals. This study demonstrated that TNBS enema was associated with these clinical and morphological changes associated with decreased body weight, increased rectal bleeding and decreased stool consistency. In this study, QCT (50 and 100 mg/kg) significantly protects against TNBS-induced colitis and it is evidenced by better clinical conditions in these test groups.
The intra-rectal instillation of TNBS has been shown to induce in massive localized erosion of the colonic mucosa leading to severe localized inflammation and hemorrhages which can be characterized by impairment in the colonic macroscopy.[18] In consistent with this report, the present investigation demonstrated that intra rectal installation of TNBS caused alteration in various parameters like colonic weight, colonic length, colonic width, colon weight/length ratio, macroscopic score, ulcer index acetic acid. Our present investigation demonstrated that QCT posttreatment significantly attenuated the alteration in colon macroscopy suggesting its role in the suppression of colitis in rats.
Oxidative stress has been implicated as a key factor in the pathogenesis of IBD both in human beings and experimental animals.[19] Sustained elevation of ROS in IBD, produced by activated phagocytic leukocytes, the xanthene oxidase pathway or oxidation of arachidonic acid overwhelm the endogenous antioxidants leading to oxidative injury, cause impairment in membrane stability and cell death.[20] Instillation of TNBS initiates production of ROS, initiating and perpetuating colonic inflammation[21] In consistent with the above reports, in the present study, noticeable alteration of lipid peroxides, nitrite/nitrate concentration and GSH levels were observed in the experimental animals after intra rectal TNBS instillation which were ameliorated by QCT/sulfasalazine treatment demonstrating an antioxidant like effect.
MPO provides a quantitative measure of disease severity and measurement of MPO activity has been used as an indicator of neutrophil influx into inflamed gastrointestinal tissue leading to release of free radicals.[22] In the present investigation, intra-rectal instillation of TNBS caused a significant elevation of MPO level which was attenuated by the QCT/sulphasalazine treatment. This ameliorative effect of can be attributed to the antioxidant like effect of QCT. GSH is one of the vital compounds for maintaining the normal cell function against ROS-induced oxidative damage.[23] Depletion of GSH promotes the generation of ROS resulting in the function and integrity of the cell. Various studies have shown that colonic GSH concentrations were significantly lowered by intra-rectal instillation of TNBS.[24] In accordance with this report, in the present study, there was a significant decrease in the GSH concentration following TNBS administration which was ameliorated by the QCT/sulphasalazine treatment confirming the antioxidant like effect.
Lipid peroxides are the secondary products of oxidative stress and produced as a result of toxic ROS produced by the administration of TNBS.[25] In the present study, intra-rectal TNBS instillation significantly increased LPO products suggesting LPO mediated damage in the colon. Treatment with QCT resulted in significant decrease in LPO levels and established its protective effect against the oxidative damage induced by TNBS. Moreover, nitrite/nitrate was also considered as an index of oxidative damage since these are oxidation products of NO. In the present investigation, we observed that the serum nitrite/nitrate levels in QCT treated animals were significantly decreased demonstrating its protective role against TNBS-induced IBD.
In conclusion, the protective effect of QCT against TNBS induced colitis can be attributed to its possible antioxidant like effect and the results suggest the therapeutic potential of QCT against TNBS-induced IBD. In the present study QCT offered dose dependent protection in the amelioration of the TNBS induced IBD like symptoms in rats, but further studies are necessary to confirm the exact mechanism of action of the protective effect of QCT against IBD like conditions in rats.
Acknowledgments
The authors are thankful to Mr. C. Janga Reddy, Chairman, Vaagdevi College of Pharmacy, Warangal, for providing the facilities required to carry out the research work. The authors are thankful to Symed Laboratories, Hyderabad, India for providing the gift sample of sulphasalazine.
Footnotes
Source of Support: Nil
Conflict Interest: No
References
- 1.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Simmonds NJ, Rampton DS. Inflammatory bowel disease - A radical view. Gut. 1993;34:865–8. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen OH, Ahnfelt-Rønne I. Involvement of oxygen-derived free radicals in the pathogenesis of chronic inflammatory bowel disease. Klin Wochenschr. 1991;69:995–1000. doi: 10.1007/BF01645145. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa DS, Cecchini R, El Kadri MZ, Rodríguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837–42. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 6.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 7.Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16:172–94. [PubMed] [Google Scholar]
- 8.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 9.Mithun VK, Amit DK, Sucheta DB. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci. 2012;4:337–43. [Google Scholar]
- 10.Ganguly AK. A method for quantitative assessment of experimentally produced ulcers in the stomach of albino rats. Experientia. 1969;25:1224. doi: 10.1007/BF01900290. [DOI] [PubMed] [Google Scholar]
- 11.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–15. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff TM, Arumugam TV, Shiels IA, Newman ML, Ross PA, Reid RC, et al. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induce colitis. Int Immunopharmacol. 2005;5:883–92. doi: 10.1016/j.intimp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–50. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 19.Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169–81. doi: 10.1016/0891-5849(92)90079-v. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–41. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J Ethnopharmacol. 2011;133:780–7. doi: 10.1016/j.jep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 23.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–21. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 24.Budarf ML, Labbé C, David G, Rioux JD. GWA studies: Rewriting the story of IBD. Trends Genet. 2009;25:137–46. doi: 10.1016/j.tig.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Kang HS, Park BE, Moon HJ, Sim SS, Kim CJ. Inhibitory effects of Geijigajakyak-Tang on trinitrobenzene sulfonic acid-induced colitis. J Ethnopharmacol. 2009;126:244–51. doi: 10.1016/j.jep.2009.08.035. [DOI] [PubMed] [Google Scholar]


