Figure 1.
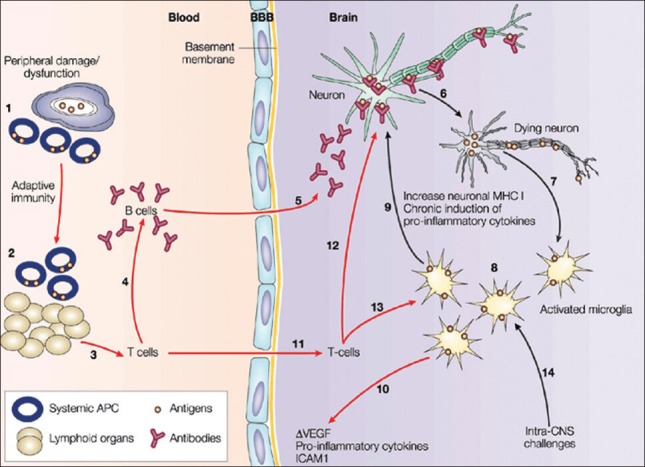
Illustration of neuronal signal transmission (see the text for details): Damage to peripheral organs initiates immune processes at the periphery, recruiting antigen-presenting cells (APCs) at the inflammatory site (1). These APCs migrate into lymphoid organs, where the transition to an adaptive immune response takes place (2). As a consequence, the clonal expansion of T-cells leads to the priming of B cells to produce antibodies (3,4). These antibodies diffuse into the central nervous system (CNS) through the blood brain barrier (BBB), or the circumventricular organs and other structures devoid of BBB (5). In the CNS, these antibodies target specific antigens and disrupt their function, causing neuronal death (6). Neuronal loss activates microglial cells that act as immune mediators in the CNS (7). The activated microglial cells phagocytose the proteins of dead neurons and present this neuronal fingerprint at their surface (8). Simultaneously, they produce pro-inflammatory cytokines and toxic molecules that compromise neuron survival (9). Eventually, in conjunction with the secretion of cytokines, microglia disturb astroglial functions, levels of vascular endothelial growth factor and intercellular adhesion molecule 1 (10), increasing the permeability of the BBB and favoring T-cell infiltration (11). T-cells have a synergistic effect on selective neuronal death by targeting neuronal antigens and priming microglia to consolidate the acquired immune response in the CNS (12,13). Alternatively, damage or pathogens within the CNS can initiate a noxious chronic innate immune response without components of systemic adaptive immunity (black arrows) (14) that can eventually promote infiltration through BBB leakage, resulting in an acquired immune response. MHC, major histocompatibility complex. Reproduced from[21] with permission from nature publishing group
