Abstract
This meta-analysis aims to examine whether the XRCC3 polymorphisms are associated with ovarian cancer risk. Eligible case-control studies were identified through search in PubMed. Pooled odds ratios (ORs) were appropriately derived from fixed effects models. We therefore performed a meta-analysis of 5,302 ovarian cancer cases and 8,075 controls from 4 published articles and 8 case-control studies for 3 SNPs of XRCC3. No statistically significant associations between XRCC3 rs861539 polymorphisms and ovarian cancer risk were observed in any genetic models. For XRCC3 rs1799794 polymorphisms, we observed a statistically significant correlation with ovarian cancer risk using the homozygote comparison (T2T2 versus T1T1: OR = 0.70, 95% CI = 0.54–0.90, P = 0.005), heterozygote comparison (T1T2 versus T1T1: OR = 1.10, 95% CI = 1.00–1.21, P = 0.04), and the recessive genetic model (T2T2 versus T1T1+T1T2: OR = 0.67, 95% CI = 0.52–0.87, P = 0.002). For XRCC3 rs1799796 polymorphisms, we also observed a statistically significant correlation with ovarian cancer risk using the heterozygote comparison (T1T2 versus T1T1: OR = 0.91, 95% CI = 0.83–0.99, P = 0.04). In conclusion, this meta-analysis shows that the XRCC3 were associated with ovarian cancer risk overall for Caucasians. Asian and African populations should be further studied.
1. Introduction
Ovarian cancer is the leading cause of the female reproductive system, with over 220,000 new cases and over 140,000 deaths worldwide in 2008 [1]. As most of the carcinomas, ovarian cancer is a multifactorial disease. Genetic factors are considered to influence the susceptibility of glioma genetic factors which all play significant roles in its susceptibility [2]. The genetic basis of ovarian carcinogenesis has been investigated in many studies. BRCA1, BRCA2, MLH1, MSH2, SMAD6, RAD51C, RAD51D, RB1, LIN28B, CASP8, and MTDH have all been implicated [3–11]. Recently, several common susceptibility alleles in four loci to be strongly associated with ovarian cancer risk have been found in three genome-wide association studies (GWAS) [12–14]. Examination of gene polymorphisms may explain individual differences in cancer risk [15].
XRCC3 (X-ray repair cross-complementing group 3) belongs to a family of genes responsible for repairing DNA double strand breaks caused by normal metabolic processes or exposure to ionizing radiation [16]. XRCC3 interacts and stabilizes Rad51 and involves in HRR (homologous recombinational repair) for DBSs (double strand breaks of DNA) and cross-link repair in mammalian cells [17, 18]. The SNP rs861539 lead to Thr241Met amino acid substitution, that may affect the function and/or its interaction with other proteins involved in DNA damage and repair [17, 19]. The SNP rs1799794 (4541 A > G) is located in 5′UTR and the SNP rs1799796 (17893 A > G) is located in intron 5 [20]. So the two SNPs do not change the proteins of XRCC3. XRCC3 polymorphism was associated with the risks of many cancers, such as lung cancer, breast cancer, and head and neck cancer [21–24]. The association between XRCC3 polymorphism and ovarian cancer has been studied [20, 25–29]; however, those experimental results remain confusing. To summarize the effect of the XRCC3 polymorphism on the risk for ovarian cancer, we performed a meta-analysis.
2. Methods
2.1. Search and Selection Process
The search of the PubMed database was performed using the following keywords: “X-ray repair cross-complementing group 3,” “XRCC3,” “rs861539,” “T241M,” “rs1799794,” “a4541g,” “rs1799796,” “a17893g,” “polymorphism,” “ovarian cancer,” and their combination. Two authors (Yuan and Wang) independently checked all the references retrieved to assess their appropriateness for the inclusion in this meta-analysis. In addition, we checked all the references cited in the articles and relevant reviews. For overlapping and republished studies, only the study with the largest samples was included. If an article reported results including different studies, each study was treated as a separate comparison in our meta-analysis.
Included studies met 3 criteria:
evaluating the association between XRCC3 polymorphisms and ovarian cancer risk;
using sufficient published data to enable estimation of an odds ratio (OR) with its 95% confidence interval (CI);
using respective or prospective cohort case-control studies.
2.2. Data Extraction
Two authors (Yuan and Wang) independently extracted data from selected articles according to the inclusion criteria and reached a consensus on all items.
The following information was extracted from each study if available: the first author, year of publication, countries, area of the cases, the ethnicity of the population, the cases source, the sample type of cases, the numbers of cases and controls, and the genotype distributions of XRCC3 in both cases and controls.
2.3. Quality Score Assessment
Two authors independently evaluated the quality of the 8 studies according to the scale for quality assessment (Table 1), which has been described previously [30, 31]. Quality score assessment was performed according to “source of cases,” “source of controls,” “specimens of cases for determining genotypes,” “Hardy-Weinberg equilibrium in controls,” and “total sample size.” Total scores ranged from 0 (worst) to 15 (best). Studies scoring ≥10 were defined as “high quality,” and those <10 were defined as “low quality.”
Table 1.
Scale for quality assessment.
| Criteria | Score |
|---|---|
| Source of cases | |
| Population or cancer registry | 3 |
| Mixed (hospital and cancer registry) | 2 |
| Hospital | 1 |
| Other | 0 |
| Source of controls | |
| Population based | 3 |
| Volunteers or Blood bank | 2 |
| Hospital based (cancer-free patients) | 1 |
| Not described | 0 |
| Specimens of cases for determining genotypes | |
| Blood or normal tissues | 3 |
| Mixed (blood and archival paraffin blocks) | 1 |
| Tumor tissues or exfoliated cells of tissue | 0 |
| Hardy-Weinberg equilibrium in controls | |
| Hardy-Weinberg equilibrium | 3 |
| Hardy-Weinberg disequilibrium | 0 |
| Total sample size | |
| ≥1000 | 3 |
| ≥500 and <1000 | 2 |
| ≥200 and <500 | 1 |
| <200 | 0 |
2.4. Statistical Analysis
Pooled ORs with 95% CIs were calculated to access the strength of association between XRCC3 polymorphism and ovarian cancer susceptibility, according to the genotype frequencies of cases and controls groups [32]. P < 0.05 was considered statistically significant; all tests and CIs were two sided. If the heterogeneity was significant, the pooled ORs were initially measured by the random effects model. Else, the fixed-effects model was chosen [33].
The XRCC3 polymorphism and ovarian cancer risk were performed for a homozygote comparison (T2T2 versus T1T1), heterozygote comparison (T1T2 versus T1T1), dominant genetic model (T1T2+T2T2 versus T1T1), and the recessive genetic model (T2T2 versus T1T1+T1T2). In addition, sensitivity analysis was performed by omitting each study. Publication bias was estimated using a funnel plot. The degree of asymmetry was examined by t Egger's test (P < 0.05 was considered significant publication bias) [34]. The analysis was carried out using Review Manager statistical software (RevMan version 5.0.17.0; The Nordic Cochrane Center, Rigshospitalet, Copenhagen, Denmark) and STATA software (version 11.2, Stata Corporation, College Station, TX, USA). Hardy-Weinberg equilibrium (HWE) was calculated using a web-based statistical tool (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
3. Results
3.1. Study Characteristics
Through the literature search, 13 articles were found. Eight articles [35–42] were excluded as irrelevant study. One study [26] was excluded because it was carried out on overlapping populations with another, more samples eligible study [27]. Total 4 articles including 8 studies were selected on 5,302 ovarian cancer cases and 8,075 controls for 3 SNPs [20, 25–27] (Figure 1). These studies were all published in English. The main characteristics of the 4 studies are shown in Table 2. All subjects in these studies were Caucasians. The sample sizes (cases and controls) ranged from 1,478 to 5,906. Quality scores for all studies were high quality (≥10). Distribution of rs861539 polymorphisms genotype frequencies among ovarian cancer cases and controls of the 2 studies is shown in Table 3. Distribution of rs1799794 polymorphisms genotype frequencies is shown in Table 4 and distribution of rs1799796 polymorphisms genotype frequencies is shown in Table 5.
Figure 1.
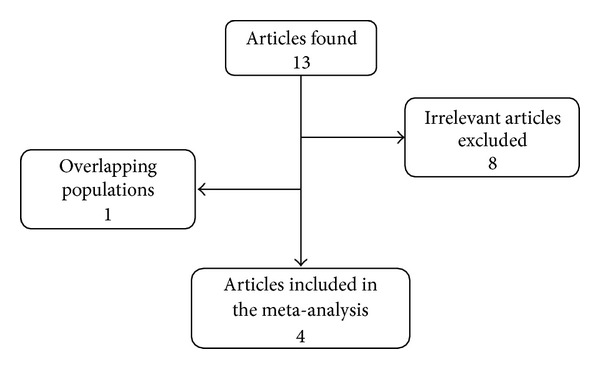
Study flow chart explaining the selection of the four articles included in the meta-analysis.
Table 2.
Main characteristics of the studies included in the meta-analysis.
| First author | Year | Country | Area of the cases | Ethnicity | Cases source | Controls source | Sample type of cases | Total cases/controls | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Auranen [20] | 2005 | Mixed (UK-USA) | Royal Marsden Hospital in London and 6 counties in Northern California | Caucasian | Mixed (hospital and cancer registry) | Population | Blood | 1665/4241 | 14 |
|
| |||||||||
| Beesley [26] | 2007 | Australia | New South Wales and Victorian Cancer Registries | Caucasian | Cancer registry | Population | Blood | 731/747 | 15 |
|
| |||||||||
| Quaye [25] | 2009 | Mixed (DK-UK-USA) | MALOVA from Denmark-SEARCH from the UK-and GEOCS from the USA. | Caucasian | Mixed (hospital and cancer registry) | Population | Blood | 1461/2299 | 14 |
|
| |||||||||
| Webb [27] | 2005 | Australia | New South Wales-Victoria and Queensland | Caucasian | Mixed (hospital and cancer registry) | Volunteers | Mixed (blood and archival paraffin blocks) | 1445/788 | 12 |
Table 3.
Distribution of XRCC3 rs861539 genotype among ovarian cancer cases and controls included in the meta-analysis.
| First author |
Year |
Genotypes distribution (Case source) |
Genotypes distribution (Controls source) |
P-HWE (Controls) |
T2T2 versus T1T1 | T1T2 versus T1T1 | T1T2+T2T2 versus T1T1 | T2T2 versus T1T1+T1T2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1T1 | T1T2 | T2T2 | T1T1 | T1T2 | T2T2 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Auranen [20] | 2005 | 676 | 762 | 227 | 1712 | 1946 | 583 | Yes | 0.99 [0.83, 1.18] | 0.88 | 0.99 [0.88, 1.12] | 0.89 | 0.99 [0.88, 1.11] | 0.87 | 0.99 [0.84, 1.17] | 0.91 |
| Beesley [26] | 2007 | 291 | 339 | 101 | 288 | 351 | 108 | Yes | 0.93 [0.67, 1.27] | 0.63 | 0.96 [0.77, 1.19] | 0.69 | 0.95 [0.77, 1.17] | 0.62 | 0.95 [0.71, 1.27] | 0.72 |
| Quaye [25] | 2009 | 545 | 612 | 175 | 784 | 958 | 282 | Yes | 0.89 [0.72, 1.11] | 0.31 | 0.92 [0.79, 1.07] | 0.27 | 0.91 [0.79, 1.05] | 0.21 | 0.93 [0.76, 1.14] | 0.51 |
| Webb [27] | 2005 | 591 | 656 | 198 | 307 | 375 | 106 | Yes | 0.97 [0.74, 1.28] | 0.83 | 0.91 [0.75, 1.10] | 0.32 | 0.92 [0.77, 1.10] | 0.37 | 1.02 [0.79, 1.32] | 0.87 |
|
| ||||||||||||||||
| Total | 2103 | 2369 | 701 | 3091 | 3630 | 1079 | 0.95 [0.85, 1.06] | 0.37 | 0.95 [0.88, 1.03] | 0.22 | 0.95 [0.88, 1.02] | 0.19 | 0.97 [0.88, 1.08] | 0.63 | ||
|
Test for
heterogeneity |
P = 0.91 |
Test for
heterogeneity |
P = 0.88 |
Test for
heterogeneity |
P = 0.82 |
Test for
heterogeneity |
P = 0.77 | |||||||||
Table 4.
Distribution of XRCC3 rs1799794 genotype among ovarian cancer cases and controls included in the meta-analysis.
| First author | Year |
Genotypes distribution (Case source) |
Genotypes distribution (Controls source) |
P-HWE (Controls) |
T2T2 versus T1T1 | T1T2 versus T1T1 | T1T2+T2T2 versus T1T1 | T2T2 versus T1T1+T1T2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1T1 | T1T2 | T2T2 | T1T1 | T1T2 | T2T2 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Auranen [20] | 2005 | 1060 | 550 | 48 | 2551 | 1188 | 161 | Yes | 0.72 [0.52, 1.00] | 0.048 | 1.11 [0.98, 1.26] | 0.087 | 1.07 [0.95, 1.20] | 0.29 | 0.69 [0.50, 0.96] | 0.027 |
| Quaye [25] | 2009 | 940 | 484 | 37 | 1505 | 713 | 89 | Yes | 0.67 [0.45, 0.99] | 0.04 | 1.09 [0.94, 1.25] | 0.25 | 1.04 [0.91, 1.19] | 0.57 | 0.65 [0.44, 0.96] | 0.027 |
|
| ||||||||||||||||
| Total | 2000 | 1034 | 85 | 4056 | 1901 | 250 | 0.70 [0.54, 0.90] | 0.005 | 1.10 [1.00, 1.21] | 0.04 | 1.06 [0.96, 1.15] | 0.24 | 0.67 [0.52, 0.87] | 0.002 | ||
|
Test for
heterogeneity |
P = 0.77 |
Test for
heterogeneity |
P = 0.83 |
Test for
heterogeneity |
P = 0.78 |
Test for
heterogeneity |
P = 0.80 | |||||||||
Table 5.
Distribution of XRCC3 rs1799796 genotype among ovarian cancer cases and controls included in the meta-analysis.
| First author | Year |
Genotypes distribution (Case source) |
Genotypes distribution (Controls source) |
P-HWE (Controls) |
T2T2 versus T1T1 | T1T2 versus T1T1 | T1T2+T2T2 versus T1T1 | T2T2 versus T1T1+T1T2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1T1 | T1T2 | T2T2 | T1T1 | T1T2 | T2T2 | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Auranen [20] | 2005 | 769 | 692 | 203 | 1757 | 1776 | 433 | Yes | 1.07 [0.89, 1.29] | 0.47 | 0.89 [0.79, 1.01] | 0.062 | 0.93 [0.83, 1.04] | 0.188 | 1.13 [0.95, 1.35] | 0.17 |
| Quaye [25] | 2009 | 676 | 608 | 177 | 1040 | 1006 | 253 | Yes | 1.08 [0.87, 1.33] | 0.5 | 0.93 [0.81, 1.07] | 0.31 | 0.96 [0.84, 1.09] | 0.536 | 1.11 [0.91, 1.37] | 0.30 |
|
| ||||||||||||||||
| Total | 1445 | 1300 | 380 | 2797 | 2782 | 686 | 1.07 [0.93, 1.24] | 0.33 | 0.91 [0.83, 0.99] | 0.04 | 0.94 [0.86, 1.03] | 0.16 | 1.13 [0.98, 1.29] | 0.08 | ||
|
Test for
heterogeneity |
P = 0.97 |
Test for
heterogeneity |
P = 0.65 |
Test for
heterogeneity |
P = 0.69 |
Test for
heterogeneity |
P = 0.90 | |||||||||
Hardy-Weinberg disequilibrium of genotype frequencies among the controls was calculated in three studies.
3.2. Association of Individual Polymorphisms with Ovarian Cancer
The heterogeneity analysis has been carried out. As it was shown in Tables 3, 4, and 5, the heterogeneities of 3 SNPs are all not significant. So the fixed-effects model was chosen for 3 SNPs.
The meta-analysis results of XRCC3 rs861539 polymorphisms are shown in Table 3. No statistically significant associations between XRCC3 rs861539 polymorphisms and ovarian cancer risk were observed in any genetic models (T2T2 versus T1T1: OR = 0.95, 95% CI = 0.85–1.06, P = 0.37; T1T2 versus T1T1: OR = 0.95, 95% CI = 0.88–1.03, P = 0.22; T1T2+T2T2 versus T1T1: OR = 0.95, 95% CI = 0.88–1.02, P = 0.19; T2T2 versus T1T1+T1T2: OR = 0.97, 95% CI = 0.88–1.08, P = 0.63).
For XRCC3 rs1799794 polymorphisms, two studies [16, 18, 20, 21, 23, 24] (3,119 cases and 6,207 controls) were eligible. The meta-analysis results of rs1799794 polymorphisms are shown in Table 4. We observed a statistically significant correlation with ovarian cancer risk using the homozygote comparison (T2T2 versus T1T1: OR = 0.70, 95% CI = 0.54–0.90, P = 0.005), heterozygote comparison (T1T2 versus T1T1: OR = 1.10, 95% CI = 1.00–1.21, P = 0.04), and the recessive genetic model (T2T2 versus T1T1+T1T2 : OR = 0.67, 95% CI = 0.52–0.87, P = 0.002). However, no statistically significant associations were observed in dominant genetic model (T1T2+T2T2 versus T1T1: OR = 1.06, 95% CI = 0.96–1.15, P = 0.24).
For XRCC3 rs1799796 polymorphisms, the meta-analysis results were shown in Table 4. We observed a statistically significant correlation with ovarian cancer risk using the heterozygote comparison (T1T2 versus T1T1: OR = 0.91, 95% CI = 0.83–0.99, P = 0.04). However no statistically significant associations were observed in homozygote comparison (T2T2 versus T1T1: OR = 1.07, 95% CI = 0.93–1.24, P = 0.33), dominant genetic model (T1T2+T2T2 versus T1T1: OR = 0.94, 95% CI = 0.86–1.03, P = 0.16), and the recessive genetic model (T2T2 versus T1T1+T1T2: OR = 1.13, 95% CI = 0.98–1.29, P = 0.08).
3.3. Publication Bias and Sensitivity Analysis
The publication bias was tested by Begg's funnel plot and Egger's test for three SNPs. Egger's test results did not show any evidence of publication bias for any of the genetic models of the three SNPs (data not shown). The shape of the four Begg's funnel plots showed no evidence of obvious asymmetry of the three SNPs (data not shown).
In the sensitivity analysis, the corresponding pooled ORs were not altered, when the fixed-effects model was changed to random-effects model. So it revealed that the results of this meta-analysis were stable.
4. Discussion
The XRCC3 gene is required for genomic stability [36]. It was reported that the XRCC3 polymorphism increased the risk of many cancers, including ovarian cancer [36]. However, the results have been inconsistent. We preformed the meta-analysis including 5,302 ovarian cancer cases and 8,075 controls for 3 SNPs of XRCC3.
For rs861539 polymorphisms, no correlation with ovarian cancer risk was observed in any genetic models. However, For XRCC3 rs1799794 and rs1799796 polymorphisms, we observed a statistically significant correlation with ovarian cancer risk. It was shown that the difference between different SNP sites was considerable for XRCC3.
All of the literature was of high quality. All study subjects were Caucasian. The global multicenter studies can provide more valuable conclusions. So further studies should be done to explore the possible relationships between XRCC3 polymorphisms and ovarian cancer risk in other ethnicities.
In conclusion, this meta-analysis shows that the XRCC3 were associated with ovarian cancer risk overall for Caucasians. Asian and African populations should be further studied.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81001166, 81272857), Independent Innovation Foundation of Shandong University (2012TS142), Natural Science Foundation of Shandong Province (ZR2010HQ050), Science and Technology Development Project of Shandong Province (2013GNC11306, czfz02), China Postdoctoral Science Foundation (201104636, 20100471551), and Soft Science Research Project of Shandong Province (2013RKE27054). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the paper.
Abbreviations
- CIs:
Confidence intervals
- HWE:
Hardy-Weinberg equilibrium
- ORs:
Odds ratios
- XRCC3:
X-ray repair cross-complementing group 3.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. Journal of the National Cancer Institute. 1998;90(23):1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 3.Meindl A, Hellebrand H, Wiek C, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nature Genetics. 2010;42(5):410–414. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 4.Loveday C, Turnbull C, Ramsay E, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nature Genetics. 2011;43(9):879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin J, Lu K, Lin J, et al. Genetic variants in TGF-β pathway are associated with ovarian cancer risk. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025559.e25559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Zhang J, Liu S, Huang Y, Chen B, Wang D. Polymorphisms in the CASP8 gene and the risk of epithelial ovarian cancer. Gynecologic Oncology. 2011;122(3):554–559. doi: 10.1016/j.ygyno.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Permuth-Wey J, Kim D, Tsai Y-Y, et al. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Research. 2011;71(11):3896–3903. doi: 10.1158/0008-5472.CAN-10-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braem MGM, Schouten LJ, Peeters PHM, van den Brandt PA, Onland-Moret NC. Genetic susceptibility to sporadic ovarian cancer: a systematic review. Biochimica et Biophysica Acta. 2011;1816(2):132–146. doi: 10.1016/j.bbcan.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Pelttari LM, Heikkinen T, Thompson D, et al. RAD51C is a susceptibility gene for ovarian cancer. Human Molecular Genetics. 2011;20(16):3278–3288. doi: 10.1093/hmg/ddr229. [DOI] [PubMed] [Google Scholar]
- 10.Ramus SJ, Antoniou AC, Kuchenbaecker KB, et al. Ovarian cancer susceptibility alleles and risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Human Mutation. 2012;33(4):690–702. doi: 10.1002/humu.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan C, Li X, Yan S, Yang Q, Liu X, Kong B. The MTDH (-470G>A) polymorphism is associated with ovarian cancer susceptibility. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051561.e51561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton KL, Tyrer J, Song H, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nature Genetics. 2010;42(10):880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goode EL, Chenevix-Trench G, Song H, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nature Genetics. 2010;42(10):874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H, Ramus SJ, Tyrer J, et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22. 2. Nature Genetics. 2009;41(9):996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zhao H, Sun L, Huang L, Yang Q, Kong B. MDM2 SNP309 is associated with endometrial cancer susceptibility: a meta-analysis. Human Cell. 2011;24(2):57–64. doi: 10.1007/s13577-011-0013-4. [DOI] [PubMed] [Google Scholar]
- 16.Tebbs RS, Zhao Y, Tucker JD, et al. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(14):6354–6358. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan P, Wang Q, Qian Q, Yu L-K. XRCC3 Thr241Met gene polymorphisms and lung cancer risk: a meta-analysis. Journal of Experimental & Clinical Cancer Research. 2013;32, article 1 doi: 10.1186/1756-9966-32-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Ye J, Li B, Ma Q, Su G, Han R. DNA repair gene XRCC3 Thr241Met polymorphism and glioma risk: a meta-analysis. International Journal of Clinical and Experimental Medicine. 2013;6(6):438–443. [PMC free article] [PubMed] [Google Scholar]
- 19.Matullo G, Palli D, Peluso M, et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and 32P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001;22(9):1437–1445. doi: 10.1093/carcin/22.9.1437. [DOI] [PubMed] [Google Scholar]
- 20.Auranen A, Song H, Waterfall C, et al. Polymorphisms in DNA repair genes and epithelial ovarian cancer risk. International Journal of Cancer. 2005;117(4):611–618. doi: 10.1002/ijc.21047. [DOI] [PubMed] [Google Scholar]
- 21.Yin Q-H, Liu C, Li L, Zu X-Y, Wang Y-J. Association between the XRCC3 T241M polymorphism and head and neck cancer susceptibility: a meta-analysis of case-control studies. Asian Pacific Journal of Cancer Prevention. 2012;13(10):5201–5205. doi: 10.7314/apjcp.2012.13.10.5201. [DOI] [PubMed] [Google Scholar]
- 22.He X-F, Wei W, Su J, et al. Association between the XRCC3 polymorphisms and breast cancer risk: meta-analysis based on case-control studies. Molecular Biology Reports. 2012;39(5):5125–5134. doi: 10.1007/s11033-011-1308-y. [DOI] [PubMed] [Google Scholar]
- 23.Tian X, Tian Y, Ma P, et al. Association between the XRCC3 C241T polymorphism and lung cancer risk in the Asian population. Tumour Biology. 2013;34(5):2589–2597. doi: 10.1007/s13277-013-0806-z. [DOI] [PubMed] [Google Scholar]
- 24.He X-F, Wei W, Li J-L, et al. Association between the XRCC3 T241M polymorphism and risk of cancer: evidence from 157 case-control studies. Gene. 2013;523(1):10–19. doi: 10.1016/j.gene.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 25.Quaye L, Tyrer J, Ramus SJ, et al. Association between common germline genetic variation in 94 candidate genes or regions and risks of invasive epithelial ovarian cancer. PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0005983.e5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beesley J, Jordan SJ, Spurdle AB, et al. Association between single-nucleotide polymorphisms in hormone metabolism and DNA repair genes and epithelial ovarian cancer: results from two Australian studies and an additional validation set. Cancer Epidemiology Biomarkers and Prevention. 2007;16(12):2557–2565. doi: 10.1158/1055-9965.EPI-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb PM, Hopper JL, Newman B, et al. Double-strand break repair gene polymorphisms and risk of breast or ovarian cancer. Cancer Epidemiology Biomarkers and Prevention. 2005;14(2):319–323. doi: 10.1158/1055-9965.EPI-04-0335. [DOI] [PubMed] [Google Scholar]
- 28.Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115(3):531–539. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearce CL, Near AM, van den Berg DJ, et al. Validating genetic risk associations for ovarian cancer through the international Ovarian Cancer Association Consortium. British Journal of Cancer. 2009;100(2):412–420. doi: 10.1038/sj.bjc.6604820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang D-K, Wang W-Z, Ren W-H, Yao L, Peng B, Yu L. TP53 Arg72Pro polymorphism and skin cancer risk: a meta-analysis. Journal of Investigative Dermatology. 2011;131(1):220–228. doi: 10.1038/jid.2010.270. [DOI] [PubMed] [Google Scholar]
- 31.Shen S-Q, Jiang D-K, Liu G-Y, Chen F, Yu L. Meta-analysis shows significant association of the TP53 Arg72Pro with ovarian cancer risk. Molecular Biology Reports. 2012;39(4):4683–4690. doi: 10.1007/s11033-011-1260-x. [DOI] [PubMed] [Google Scholar]
- 32.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen BS, Konstantinopoulos PA, Spillman MA, De S. Copy neutral loss of heterozygosity is more frequent in older ovarian cancer patients. Genes Chromosomes and Cancer. 2013;52(9):794–801. doi: 10.1002/gcc.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng CX, Xue M, Li K, Li WS. Predictive value of XRCC1 and XRCC3 gene polymorphisms for risk of ovarian cancer death after chemotherapy. Asian Pacific Journal of Cancer Prevention. 2012;13(6):2541–2545. doi: 10.7314/apjcp.2012.13.6.2541. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Hormazabal P, Reyes JM, Blanco R, et al. The BARD1 Cys557Ser variant and risk of familial breast cancer in a South-American population. Molecular Biology Reports. 2012;39(8):8091–8098. doi: 10.1007/s11033-012-1656-2. [DOI] [PubMed] [Google Scholar]
- 38.Clague J, Wilhoite G, Adamson A, Bailis A, Weitzel JN, Neuhausen SL. RAD51C germline mutations in breast and ovarian cancer cases from high-risk families. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025632.e25632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drew Y, Mulligan EA, Vong W-T, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. Journal of the National Cancer Institute. 2011;103(4):334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 40.Gottipati P, Vischioni B, Schultz N, et al. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Research. 2010;70(13):5389–5398. doi: 10.1158/0008-5472.CAN-09-4716. [DOI] [PubMed] [Google Scholar]
- 41.Jakubowska A, Gronwald J, Menkiszak J, et al. BRCA1-associated breast and ovarian cancer risks in Poland: no association with commonly studied polymorphisms. Breast Cancer Research and Treatment. 2010;119(1):201–211. doi: 10.1007/s10549-009-0390-5. [DOI] [PubMed] [Google Scholar]
- 42.Danoy P, Sonoda E, Lathrop M, Takeda S, Matsuda F. A naturally occurring genetic variant of human XRCC2 (R188H) confers increased resistance to cisplatin-induced DNA damage. Biochemical and Biophysical Research Communications. 2007;352(3):763–768. doi: 10.1016/j.bbrc.2006.11.083. [DOI] [PubMed] [Google Scholar]


