Figure 1.
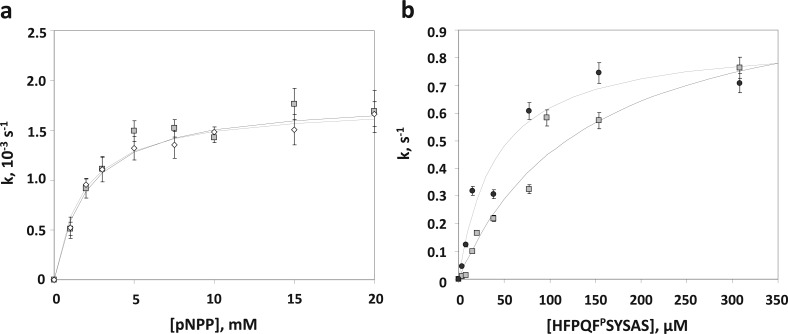
PHLPP activity is highly dependent on the substrate. (a) PHLPP1 and PHLPP2 have similar phosphatase activities in vitro. The saturating kinetics of the phosphatase domains of PHLPP1 (empty diamonds) and PHLPP2 (gray squares) with increasing concentrations of the substrate pNPP is shown. The rates were determined by measuring the production of pNP at 405 nm in Tricine buffer (pH 7.5). The data are fit to the Michaelis–Menten equation. The graph shows mean values ± SEM for three separate experiments. (b) Activities of the phosphatase domain of PHLPP2 isolated from bacteria or insect cells are comparable on peptidic substrates. The saturating kinetics of the phosphatase domains of PHLPP2 isolated from Sf21 cells (black circles) or Escherichia coli BL21(DE3)pLysS (gray squares) with increasing concentrations of the substrate peptide is shown. The rates were determined by measuring the liberation of free phosphate by the malachite green assay in a Tricine buffer (pH 7.5). The data are fit to the Michaelis–Menten equation. The graph shows mean values ± SEM for three separate experiments.