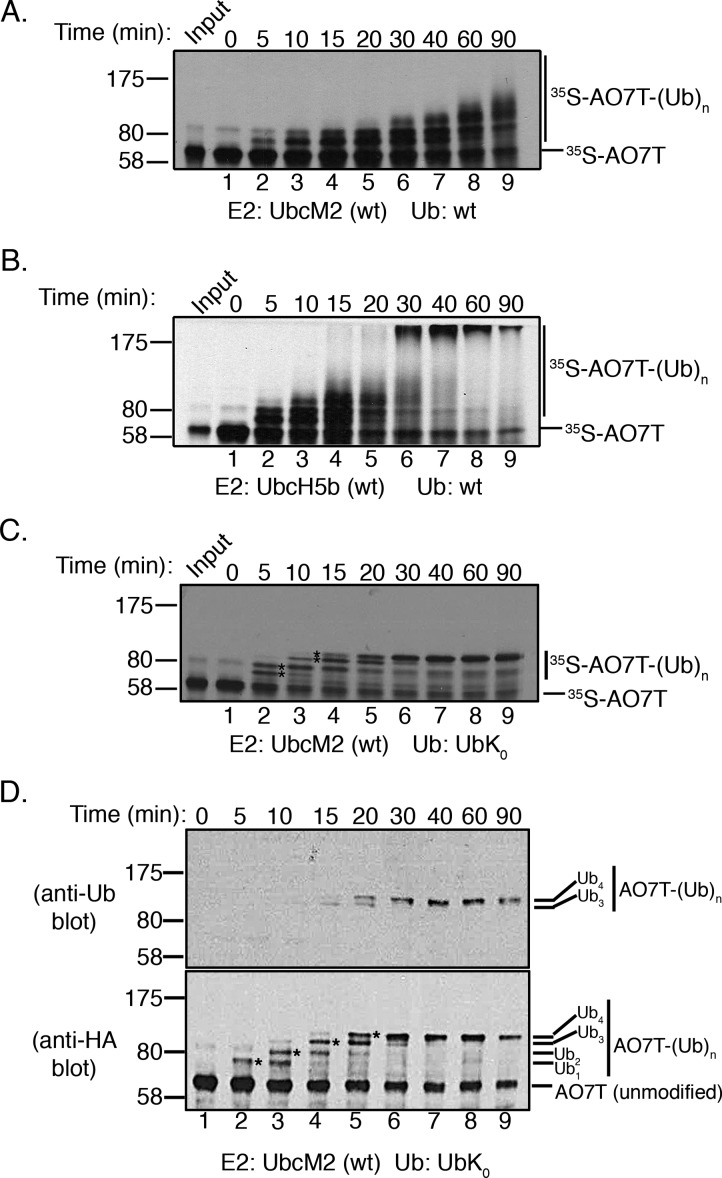
Figure 1.
UbcM2 has a severely limited capacity to synthesize polyUb chains on AO7T with wt Ub. (A) Fully recombinant in vitro ubiquitylation assays containing recombinant E1, H6T-UbcM2, wt Ub, energy, and [35S]AO7T (produced in a bacterial TNT expression system) were incubated at 37 °C for the indicated times. Reaction mixtures were solubilized and processed for SDS–PAGE and fluorography. (B) Same as panel A except testing H6T-UbcH5b. For panels A and B, [35S]AO7T input was loaded to the left of lane 1 and the migration of molecular weight markers is denoted on the left. Unmodified [35S]AO7T is marked on the right of each fluorograph with a hashmark and Ub-modified [35S]AO7T [[35S]AO7T-(Ub)n] with a vertical line. (C) Assay similar to that in panel A except wt Ub has been replaced with lysine-less Ub (UbK0). Distinct bands representing the attachment of one, two, three, or four monoUbK0 molecules to AO7T are denoted with asterisks between lanes 2 and 3 and lanes 3 and 4. (D) Assay similar to that in panel C except that HA-AO7T is not radiolabeled with [35S]Met/Cys but rather reaction products are visualized by either anti-Ub (top) or anti-HA Western blotting (bottom). The number of UbK0 molecules conjugated to HA-AO7T is indicated to the right of each blot. The sensitivity of the anti-Ub antibody is such that only HA-AO7T modified with either three or four UbK0 molecules is detected. All experiments were repeated at least three independent times.