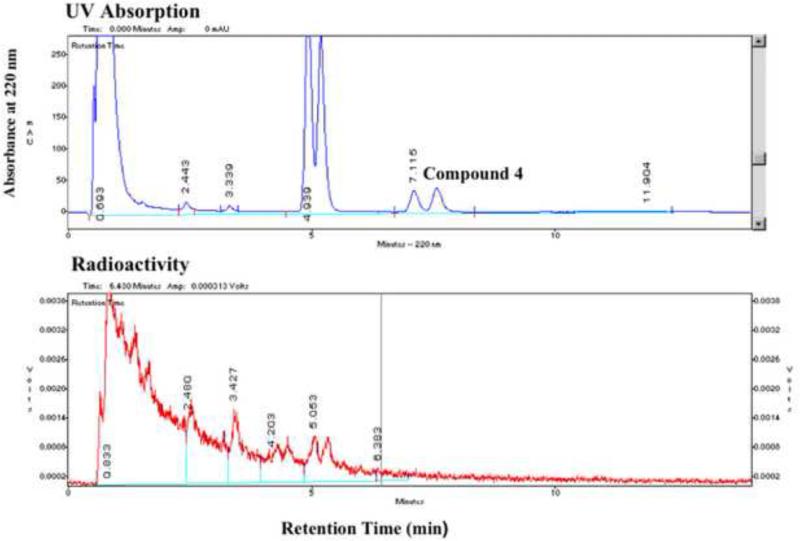
Figure 2a.
HPLC analysis of a reaction between the OH-protected ClCF2-precursor (4, Approach A) with K18F/Kryptofix in DMF after heating at 150°C for 10 minutes. The analytical HPLC was performed using a C18 column and a mobile phase of acetonitrile and water (40/60) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). Compound 4, which is a mixture of 2 diastereomers, appears as a double peak in the HPLC chromatogram. The major product of the reaction, which appears as a double peak at a retention time of approximately 5 min, is also a mixture of 2 diastereomers (see Scheme 1). (Vertical axes are in arbitrary units.)