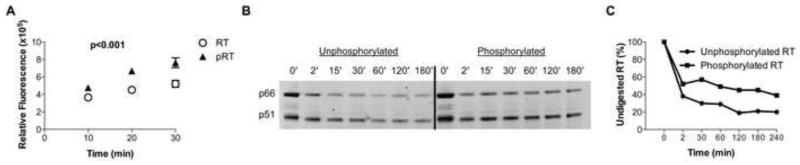
Figure 4. CDK2-dependent phosphorylation enhances activity and stability of HIV-1 RT.
(A) Assessment of the functional activity of phosphorylated and unphosphorylated HIV-1 RT in a cell-free system. One representative experiment out of 4 is shown. (B) SDS-PAGE gel of limited proteolysis of unphosphorylated (left) and cyclin A/CDK2 phosphorylated (right) HIV-1 RT time course. Degradation of the p66 and p51 subunits of HIV-1 RT (major upper and lower bands, respectively) is shown as a function of time and phosphorylation state. (C) Quantitative comparison of the rates of proteolysis for unphosphorylated and phosphorylated HIV-1 RT as measured by SDS-PAGE gel densitometry of Sypro Ruby-stained HIV-1 RT. See also Figures S3.