The global incidence of fatty liver disease (FLD) has eclipsed nearly every other hepatic pathology, largely due to the obesity epidemic. Steatosis is the first requisite step in progression to steatohepatitis and cirrhosis, the more severe forms of FLD. Lifestyle changes remain the most effective treatment for FLD; however, waistlines continue to expand in parallel with a booming weight-loss industry, indicating that lifestyle changes are both difficult to achieve and ineffective for many patients. Thus, targets for therapeutic intervention are sought. A study in this issue of Hepatology 1 suggests a novel pathway that contributes to steatosis, opening new avenues for designing drugs to alleviate FLD.
Many investigators studying hepatic injury have focused on the role of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2). High levels of ROS can result in severe cell dysfunction or death. Some studies suggest that ROS can cause FLD, while many others focus on the consequence of ROS once FLD has formed. Promising results from clinical trials using antioxidants in patients with nonalcoholic2 and alcoholic3 FLD (i.e., nonalcoholic fatty liver disease [NAFLD] and alcoholic liver disease [ALD], respectively) support the theory that ROS can cause steatosis as well as the hepatic injury associated with FLD. The work by Nussbaum et al.1 in this issue of Hepatology challenges this theory by demonstrating a paradoxic role for ROS in FLD: instead of contributing to the pathogenesis of FLD, low (i.e., homeostatic) levels of ROS appear to prevent steatosis and addition of antioxidants promotes steatosis. Moreover, they uncover a previously unrecognized link between purine metabolism, ROS, and regulation of lipid droplet formation and utilization in hepatocytes.
This study benefited from the use of zebrafish, a small vertebrate with high reproductive capacity and a fully sequenced genome that shows high conservation with humans.4 The larval liver has a cellular composition comparable to mammals, and their hepatocytes can synthesize, store, and metabolize lipids. Thus, zebrafish, like mammals, get FLD.5 These attributes make zebrafish the only vertebrate amenable to forward genetic screening, which provides an unbiased approach to identify novel genes involved in FLD. Additionally, zebrafish larvae are transparent, providing an unparalleled view inside of the growing organism. The authors capitalize on this feature to develop a novel means to measure steatosis. Confocal imaging of the liver sections of larvae, where the contours of the hepatocytes are outlined with green fluorescent protein (GFP) and a fluorescent dye that labels the lipid droplets, allows quantification of cells containing lipid droplets as well as the number of droplets per cell, and also generates exquisite 3D images, rendering this pathology at subcellular resolution.
The mechanisms of steatosis can be simplistically viewed as an imbalance in four processes: excessive lipid synthesis or import, or reduced lipid utilization or export. Reverse genetic approaches, whereby a gene of interest is selected, specifically targeted, and the effect on hepatic lipid accumulation is evaluated, have provided a detailed understanding of how the core machinery of the lipid metabolism is regulated in hepatocytes, and how these processes are disrupted in FLD. While this approach is highly valuable, it does not facilitate discovery of entirely novel processes that impact lipid metabolism in hepatocytes.
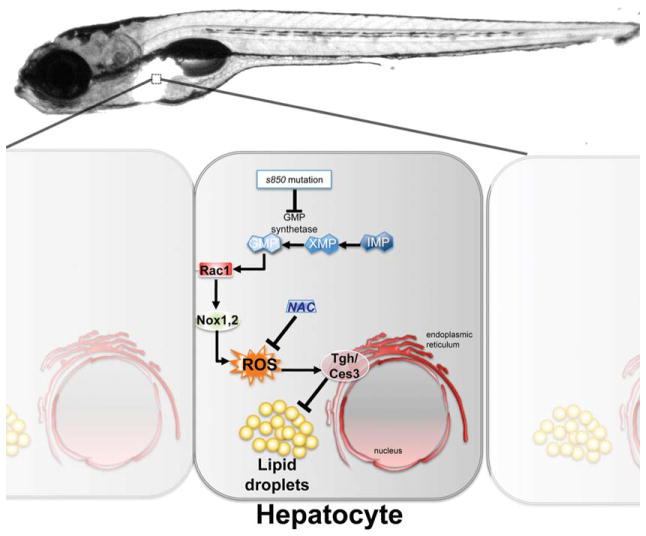
Enter zebrafish—a large-scale forward genetic screen in zebrafish was carried out to identify mutants with liver defects,6 and the current study identifies one of these mutants to develop steatosis by 7 days postfertilization. The first unexpected result demonstrates that a mutation in the gene encoding guanosine 5′-monophosphate (GMP) synthetase (gmps), a key enzyme in purine metabolism, leads to steatosis. De novo nucleotide synthesis is a major hepatocyte function and is stimulated in response to insulin; however, the link between the purine synthesis and the hepatic lipid metabolism had not been described previously. This study dissects the complex pathway outlined in Fig. 1, whereby a loss of GMP reduces Rac1 activity and homeostatic ROS production, which then lead to a reduction of carboxylesterase (ces3; also called triglyceride hydrolase [tgh]) that cleaves triglycerides stored in hepatocytes as lipid droplets.
Fig. 1.
Homeostatic levels of ROS are maintained by GMP levels and are required to prevent steatosis in zebrafish larvae. This simplified schematic illustrates how de novo purine synthesis is linked to hepatic lipid metabolism as revealed by the s850 mutation in the GMP synthetase gene. Adequate supply of GMP in hepatocytes maintains Rac1 in its active form, which indirectly induces homeostatic generation of ROS by way of Nox1,2. This, in turn, promotes activation of Tgh/Ces3, the hydrolase that releases triglycerides from lipid droplets. Inhibition of expression or activity of these players, including blocking ROS by using NAC, induces steatosis. GMP, guanosine monophosphate; IMP, inosine monophosphate; NAC, N-acetyl cysteine; Nox, NADPH oxidase; ROS, reactive oxygen species; Tgh/Ces3, triglyceride hydrolase/carboxylesterase3; XMP, xanthosine monophosphate.
Their second novel finding shows that loss of Rac1 blocks production of homeostatic ROS. Rac1 is a small GTPase best studied in the context of cytoskeletal rearrangements in response to signaling from cell surface receptors. A recent study suggested that Rac1 activation could induce the JNK pathway, a major player in hepatic injury, as JNK activation could lead to apoptosis in hepatocytes7 and cause steatosis. 8 This tenuous connection may provide a mechanistic link between Rac1 activation and steatosis. Interestingly, an alternative possibility is provided by the discovery that gmps mutation reduces Rac1 activity and that both pharmacologic and genetic inhibition of Rac1 in wild-type zebrafish larvae is sufficient to induce steatosis.1 These findings implicate GMP as a novel regulator of Rac1 activity and suggest that Rac1 activation prevents FLD. Whether JNK plays a part in this pathway remains an outstanding question.
The third and the most surprising finding is that homeostatic ROS prevent steatosis. Cells possess elaborate, potent antioxidant mechanisms to protect against cellular damage caused by excessive ROS. The DNA and protein adducts as well as organelle damage that are characteristic of oxidative stress occur when ROS levels overwhelm the cellular antioxidant defense system. However, a growing body of literature indicates that at low (i.e., homeostatic) levels, ROS serve as an important signaling molecule in processes as diverse as cell proliferation, metabolism, differentiation, survival, antiinflammatory responses, iron homeostasis, and DNA repair.9 Nussbaum et al. add regulation of lipid droplets to this list by reporting that low levels of H2O2 prevent steatosis in gmps mutants, and that antioxidant supplementation to wild-type larvae reduces homeostatic ROS and induces steatosis. Whether modulating homeostatic ROS in humans by antioxidant treatment affects steatosis has yet to be evaluated.
How does disrupting the GMP-Rac1 axis contribute to lipid accumulation in hepatocytes? Tantalizing evidence of deregulation of lipid droplet hydrolysis as a mechanism for steatosis is proposed by their finding that ces3 was downregulated in larvae deficient for the GMP synthesis-Rac1 axis. Moreover, simply treating larvae with a low dose of H2O2 restores ces3 expression in gmps mutants. This contrasts with a recent study using mice deficient for Ces3/Tgh, which had a marked decrease in steatosis in both fasted and fed states. Pharmacologic inhibition of Tgh reduced lipid turnover in primary human hepatocytes,10 suggesting that Ces3 reduction could alternatively reduce or increase lipid droplet formation in different contexts. It will be interesting to delineate whether Tgh/Ces3 regulation is a genuine and conserved factor that prevents steatosis across species.
This work highlights a number of intriguing issues that may inform clinical practice. For instance, there are substantial data that support the use of antioxidants to reduce liver injury in FLD patients. However, it is possible that antioxidants also suppress the generation of homeostatic levels of ROS, which may reduce tgh/ces3 expression and, as a consequence, prevent hydrolysis of lipid droplets, leading to steatosis. Also, given that GMP appears an important factor in regulating lipid droplet formation, it is exciting to speculate that supplementation of this nucleotide may serve to suppress this pathway in patients. Finally, this work provides an illustrative example of how using unbiased screening, and unconventional models, can generate surprising and novel ideas that advance our understanding of FLD and provide new areas to exploit for therapeutic intervention.
Abbreviations
- ALD
alcoholic liver disease
- FLD
fatty liver disease
- GMP
guanosine 5′-monophosphate
- NAFLD
nonalcoholic fatty liver disease
- ROS
reactive oxygen species
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Nussbaum J, Hasan S, Liu L, Schaub M, McClendon A, Stainier DY, et al. Homeostatic generation of reactive oxygen species protects zebrafish liver from hepatic steatosis. Hepatology. 2013;58:1326–1338. doi: 10.1002/hep.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen-Khac E, Thevenot T, Piquet M-A, Benferhat SØ, Goria O, Chatelain D, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 4.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013 doi: 10.1038/nature12111. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–3572. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 6.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56:192–198. doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 9.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, et al. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]