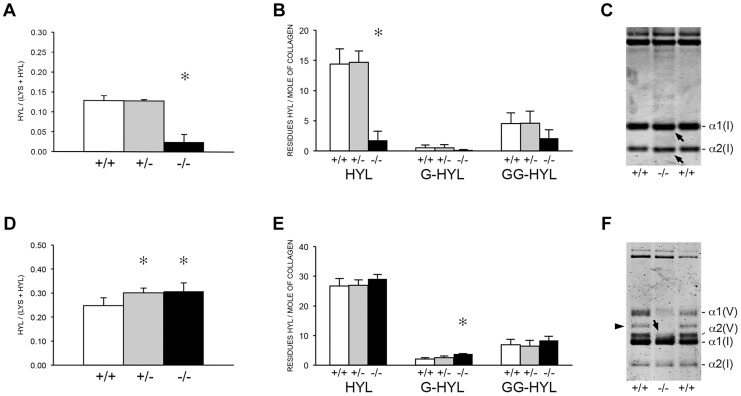
Figure 6. Post-translational modification of type I collagen from tissues.
(A) Total lysyl hydroxylation of type I collagen is dramatically reduced in dermal tissue from Ppib −/− mice (p<0.001 vs wild-type). (B) Post-translational hydroxylation and glycosylation of type I collagen lysyl residues in dermal tissue from Ppib −/− mice. (C) SDS-Urea PAGE analysis of pepsin extracts from dermal tissue demonstrates increased electrophoretic migration of Ppib −/− type I collagen alpha chains compared to wild-type. Arrows indicate faster migrating alpha chains. (D) Total lysyl hydroxylation of type I collagen extracted from bone tissue of heterozygous (+/−) and homozygous (−/−) Ppib-null mice compared to wild-type (+/+) bone collagen. Both Ppib +/− and Ppib −/− bone collagen show increased Hyl compared to wild-type (p = 0.0002 and 0.005, respectively). (E) Analysis of post-translational lysine hydroxylation and glycosylation in Ppib −/− bone-derived type I collagen demonstrates increased galactosyl-hydroxylysine (G-HYL) content compared to wild-type (p<0.001). (F) Type I collagen extracted from bone tissue displays backstreaking of α1(I) chains on SDS-Urea, indicated by arrow, and is consistent with post-translational overmodification. Arrowhead indicates a truncated form of α1(V) chains due to pepsin sensitivity.