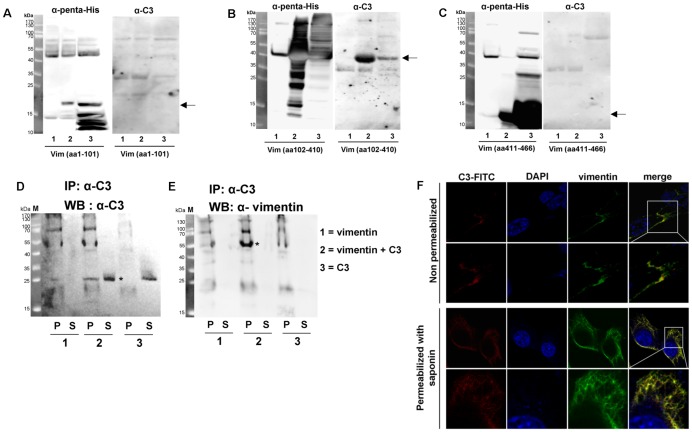
Figure 3. Binding of C3 to the rod domain of vimentin.
His-tagged vimentin domains (A) head: amino acids 1 to 101; (B) rod: amino acids 102 to 410; (C) tail: amino acids 411 to 466 were expressed in E. coli. Crude extracts from non-induced E. coli (lane 1), crude extracts from induced E. coli (lane 2) and His-tagged vimentin domains purified through Ni-IDA column (lane 3) were separated by 15% SDS-PAGE and electroblotted. Left panels of A, B and C show probing with penta-His polyclonal antibody to detect vimentin domains. Right panels of A, B and C show probing with anti-C3 to detect bound C3. Predicted apparent mol masses of each vimentin domain are indicated by arrow. Arrows indicate the protein of interest. D) Immunoprecipitation of C3 with C3 antibody. Full length recombinant vimentin was incubated with C3 in solution. The vimentin-C3 complex was immunoprecipitated with C3 antibody. P = pellet of agarose beads, S = supernatant. E) Immunoprecipitation of vimentin with C3 antibody and detection of vimentin. P = pellet of agarose beads, S = supernatant. F) For imaging vimentin and C3 at the cell surface (upper panel), cells were incubated with C3-FITC for 1 h at 4°C. After washing with PBS cells were fixed (without permeabilization) and incubated with vimentin antibody following by an Alexa-555 conjugated secondary antibody. For imaging intracellular vimentin (lower panel), cells were fixed, permeabilized and incubated as described above. For imaging intracellular C3, living cells were incubated with C3-FITC for 1 h at 37°C. After washing with PBS cells were fixed, permeabilized with 0.02% saponin for 30 min and imaged by confocal microscopy.