Abstract
Background
Low-volume high-intensity interval training (HIT) appears to be an efficient and practical way to develop physical fitness.
Objective
Our objective was to estimate meta-analysed mean effects of HIT on aerobic power (maximum oxygen consumption [VO2max] in an incremental test) and sprint fitness (peak and mean power in a 30-s Wingate test).
Data Sources
Five databases (PubMed, MEDLINE, Scopus, BIOSIS and Web of Science) were searched for original research articles published up to January 2014. Search terms included ‘high intensity’, ‘HIT’, ‘sprint’, ‘fitness’ and ‘VO2max’.
Study Selection
Inclusion criteria were fitness assessed pre- and post-training; training period ≥2 weeks; repetition duration 30–60 s; work/rest ratio <1.0; exercise intensity described as maximal or near maximal; adult subjects aged >18 years.
Data Extraction
The final data set consisted of 55 estimates from 32 trials for VO2max, 23 estimates from 16 trials for peak sprint power, and 19 estimates from 12 trials for mean sprint power. Effects on fitness were analysed as percentages via log transformation. Standard errors calculated from exact p values (where reported) or imputed from errors of measurement provided appropriate weightings. Fixed effects in the meta-regression model included type of study (controlled, uncontrolled), subject characteristics (sex, training status, baseline fitness) and training parameters (number of training sessions, repetition duration, work/rest ratio). Probabilistic magnitude-based inferences for meta-analysed effects were based on standardized thresholds for small, moderate and large changes (0.2, 0.6 and 1.2, respectively) derived from between-subject standard deviations (SDs) for baseline fitness.
Results
A mean low-volume HIT protocol (13 training sessions, 0.16 work/rest ratio) in a controlled trial produced a likely moderate improvement in the VO2max of active non-athletic males (6.2 %; 90 % confidence limits ±3.1 %), when compared with control. There were possibly moderate improvements in the VO2max of sedentary males (10.0 %; ±5.1 %) and active non-athletic females (3.6 %; ±4.3 %) and a likely small increase for sedentary females (7.3 %; ±4.8 %). The effect on the VO2max of athletic males was unclear (2.7 %; ±4.6 %). A possibly moderate additional increase was likely for subjects with a 10 mL·kg−1·min−1 lower baseline VO2max (3.8 %; ±2.5 %), whereas the modifying effects of sex and difference in exercise dose were unclear. The comparison of HIT with traditional endurance training was unclear (−1.6 %; ±4.3 %). Unexplained variation between studies was 2.0 % (SD). Meta-analysed effects of HIT on Wingate peak and mean power were unclear.
Conclusions
Low-volume HIT produces moderate improvements in the aerobic power of active non-athletic and sedentary subjects. More studies are needed to resolve the unclear modifying effects of sex and HIT dose on aerobic power and the unclear effects on sprint fitness.
Introduction
High-intensity interval training (HIT), which involves alternating bouts of intensive exercise with low-intensity recovery periods [1], is considered one of the most effective means of improving cardiorespiratory and metabolic function [2]. Athletes and coaches have historically used HIT to improve exercise performance, but the effectiveness of HIT to improve health-related outcomes has recently generated new interest [3]. In recent reviews, there appears to be a consensus for the benefit of high-intensity aerobic interval training in patient populations [3–6]. Weston et al. [6] meta-analysed ten studies and reported that high-intensity aerobic interval training, typically performed at 85–95 % maximal heart rate (%HRmax), increased cardiorespiratory fitness by almost double that of moderate-intensity continuous training in patients with lifestyle-induced chronic disease. In contrast, HIT of similar intensity elicits improvements in maximal oxygen uptake (VO2max) slightly greater than is typically reported with continuous training in healthy, active adults [7].
HIT can encompass a considerable range of exercise intensities. For example, Buchheit and Laursen [8] recently defined HIT as “either repeated short (<45 s) to long (2–4 min) bouts of rather high- but not maximal-intensity exercise, or short (<10 s, repeated-sprint sequences) or long (>20–30 s, sprint interval session) all-out sprints, interspersed with recovery periods.” As such, maximal, all-out sprint training is classified as a form of high-intensity training at the highest end of the intensity spectrum [9, 10]. Here, the repeated bouts of relatively brief all-out (maximal) intermittent exercise necessitate shorter interval durations and longer recovery periods than those of traditional high-intensity aerobic interval programming, and the total weekly volume (duration) of exercise is therefore lower. There is accumulating evidence supporting improved aerobic exercise performance following this form of training. Kessler et al. [3] reviewed five studies with exercise intensity described as all-out and concluded that it was an effective means of improving VO2max. Sloth et al. [10] meta-analysed standardized effects of low-volume all-out interval training on VO2max in 13 studies and reported an overall moderate effect (standardised change in the mean of 0.63). However, their meta-analysis did not account for the modifying effects of study and subject characteristics, or studies with reference groups representing traditional endurance training, rather than no training. Using similar inclusion criteria (e.g. 30-s all-out sprints) Gist and colleagues [11] meta-analysed 16 randomized controlled trials and reported a moderate effect (0.69) of HIT on VO2max in comparison with no-exercise control groups and a trivial effect (0.04) when compared with endurance-training controls. However, effects on physical performance should be meta-analysed in percent units before assessment via standardization [12]. Gist et al. [11] reported no significant effects of initial fitness, intervention length, inclusion of additional training or mode of training in response to HIT, but they did not report the effect of sex or work to rest ratio. The magnitude of the benefit of low-volume HIT on aerobic power, therefore, has still to be summarised adequately.
Low-volume HIT may also have the potential to improve sprint power, as it increases enzymatic activities of anaerobic metabolism [13]. Most sporting activities depend upon the expression of power for short or sustained periods of time [14]. Furthermore, many basic daily activities are dependent on the ability to generate force at high velocity, and power training can improve mobility-related outcomes in the elderly [15] as well as increasing self-efficacy, satisfaction with physical function and overall life satisfaction [16]. A meta-analysis of the effect of HIT on sprint power is therefore timely. Our aim for this review was to use a mixed-model meta-analysis to provide estimates of the effect of low-volume HIT on fitness (VO2max, 30-s Wingate power) along with the modifying effects of study and subject characteristics.
Methods
Literature Search
A search of five databases (PubMed, MEDLINE, Scopus, BIOSIS and Web of Science), along with the reference lists of original research and review articles published in English up to January 2014 was conducted by two of the authors (KT, MW). Our independent variable search terms were ‘aerobic high intensity’, ‘high intensity’, ‘HIT’, ‘intervals’, ‘intensive’, ‘sprint’, ‘repeated sprint’, and the dependent variable search terms were ‘fitness’, ‘aerobic fitness’, ‘anaerobic fitness’, ‘VO2max’ ‘performance’, ‘endurance’ and ‘adaptations’. Independent variable search terms were combined with dependent variable search terms, giving a total of 49 combinations.
Study Selection
The most common model employed in low-volume HIT studies consisted of four to six 30-s ‘all-out’ efforts separated by ~4 min of recovery, for a total of two to three minutes of intense exercise during a single training session. As such, our study selection criteria were VO2max or 30-s Wingate power assessed pre- and post-training, training period ≥2 weeks, repetition duration 30–60 s, work/rest ratio <1.0, exercise intensity described as maximal or near maximal, and adult subjects aged >18 years. No inclusion criteria were used for participant fitness. Using the subject characteristic information provided by each study, participants were assigned to one of three groups: sedentary, active non-athletic or athletic.
The selection of studies for our meta-analysis was confined to studies predominantly utilizing the classic Wingate protocol. In doing so, we acknowledge the exclusion of a large body of laboratory- and field-based HIT research utilizing longer interval durations (1–4 min) performed at high, but not maximal intensity, and with a work:rest ratio ≥1.0 [8]. Furthermore, by selecting VO2max as our measure of aerobic fitness, we excluded field-relevant performance measures, which may limit the application of our findings to athletic populations and sports performance. The recent meta-analysis by Bacon et al. [7] has, to an extent, addressed this gap in the literature. However, the number of studies excluded from the present study on interval duration, intensity and other measures of aerobic fitness (e.g. velocity at VO2max, speed at lactate threshold, running economy and sports-specific tests) underscores the need for a dedicated review of studies using longer intervals at lower intensities.
To select relevant papers, all titles were initially screened by two authors (KT, MW) during the electronic searches to exclude studies that were beyond the scope of this meta-analysis. Following this initial selection process, there were 550 potentially eligible studies (Fig. 1). All study titles and abstracts were then screened independently by the same authors. Full-text versions of the remaining papers that met each of the eligibility criteria were then reviewed by these authors to determine final inclusion in the meta-analysis. Any disputed studies were taken to a third reviewer (AMB) for resolution. The final dataset for VO2max consisted of 55 estimates from 32 trials, 11 of which were controlled trials. For peak sprint power, the final dataset consisted of 23 estimates from 16 trials, three of which were controlled trials. For mean sprint power, the dataset consisted of 19 estimates from 12 trials, three of which were controlled trials.
Fig. 1.
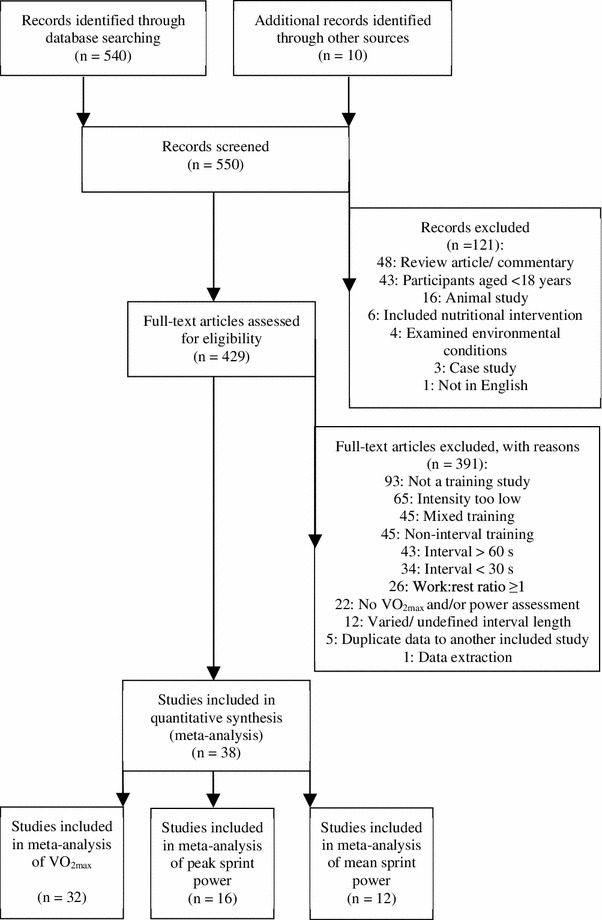
Flow diagram of study selection. VO2max maximum oxygen consumption
Data Extraction
Graph digitizer software (DigitizeIt, Germany) was used to obtain data values in studies where only plots were published. Accuracy was confirmed via intra- and inter-individual reassessments of data extraction. Mean effects on VO2max, peak and mean sprint power in training and control groups were converted to a percentage change. For each converted effect, standard errors were calculated to indicate the level of imprecision. In studies where exact p values were given (VO2maxn = 7; peak power n = 4; mean power n = 5), standard errors were calculated directly via the corresponding t statistic and its degrees of freedom. Under the assumption that studies with similar test protocols and subject characteristics would have similar typical errors of measurement, the typical errors from these studies were then averaged (via the weighted mean variance) and assigned to the studies that did not report an exact p value. The standard error was then calculated via the relationship between typical error and standard error [17, 18]. Descriptive statistics for studies included in the meta-analysis for VO2max, peak and mean sprint power are shown in Tables 1, 2 and 3, respectively.
Table 1.
Study and subject characteristics for maximum oxygen consumption estimates included in the meta-analysis
| References | Study design | Subjects | Mean age, y | Sample size | Proportion of males | Duration (weeks) | Total sessions | Group | Exercise intensitya | No. of reps | Total reps | Rep duration (s) | Work/rest ratio | Effect on VO2max (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Mean | SE | |||||||||||||
| Rakobowchuk et al. [20] | NC | Non-ath | 23.1 | 11 | 0.36 | 6 | 18 | HIT | 100 %Pmax | 20 | 27 | 420 | 30 | 0.50 | 15.4 | 2.8 |
| Siahkouhian et al. [21] | NC | Non-ath | 19.1 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 7 | 191 | 30 | 0.13 | 13.9 | 3.0 |
| Siahkouhian et al. [21] | NC | Ath | 19.4 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 7 | 191 | 30 | 0.13 | 7.3 | 3.0 |
| Trilk et al. [22] | C | Sed | 30.1 | 14 | 0.00 | 4 | 12 | HIT | All-out | 4 | 7 | 66 | 30 | 0.13 | 13.4 | 2.5 |
| Trilk et al. [22] | C | Sed | 31.4 | 14 | 0.00 | 4 | – | CON | – | – | – | – | – | – | −0.5 | 2.5 |
| Allemeier et al. [23] | C | Non-ath | 22.7 | 11 | 1.00 | 6 | 15 | HIT | All-out | 3 | 3 | 45 | 30 | 0.03 | 12.5 | 2.8 |
| Allemeier et al. [23] | C | Non-ath | 24.0 | 6 | 1.00 | 6 | – | CON | – | – | – | – | – | – | −0.7 | 3.8 |
| McKenna et al. [24] | NC | Non-ath | 20.9 | 8 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 12.5 | 3.3 |
| Macpherson et al. [25] | NC | Non-ath | 24.3 | 10 | 0.60 | 6 | 18 | HIT | All-out | 4 | 6 | 90 | 30 | 0.13 | 11.5 | 2.9 |
| Macpherson et al. [25] | NC | Non-ath | 22.8 | 10 | 0.60 | 6 | 18 | END | 65 %VO2max | – | – | – | 2,700 | – | 12.5 | 2.9 |
| Esfandiari et al. [26] | NC | Non-ath | 24.5 | 8 | 1.00 | 2 | 6 | HIT | 95–100 %VO2max | 8 | 12 | 60 | 60 | 0.8 | 11.1 | 3.7 |
| Esfandiari et al. [26] | NC | Non-ath | 25.6 | 8 | 1.00 | 2 | 6 | END | 65 %VO2max | – | – | – | 6,300 | – | 4.5 | 3.7 |
| Dunham and Harms [27] | NC | Non-ath | 20.2 | 8 | ? | 4 | 12 | HIT | 90 %Pmax | 5 | 5 | 60 | 60 | 0.33 | 9.6 | 3.7 |
| Dunham and Harms [27] | NC | Non-ath | 21.3 | 7 | ? | 4 | 12 | END | 60–70 %Pmax | – | – | – | 2,700 | – | 5.5 | 4.0 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | All-out | 3 | 4 | 48 | 30 | 0.13 | 9.6 | 4.0 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | 125 %Pmax | 6 | 8 | 96 | 30 | 0.25 | 9.7 | 4.1 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | – | CON | – | – | – | – | – | – | −0.9 | 5.5 |
| Whyte et al. [29] | NC | Sed | 32.1 | 10 | 1.00 | 2 | 6 | HIT | All-out | 4 | 6 | 30 | 30 | 0.11 | 9.5 | 3.2 |
| Hazell et al. [30] | C | Non-ath | 24.0 | 12 | 0.73 | 2 | 6 | HIT | All-out | 4 | 6 | 30 | 30 | 0.13 | 9.3 | 2.7 |
| Hazell et al. [30] | C | Non-ath | 24.0 | 12 | ? | 2 | – | CON | – | – | – | – | – | – | 0.2 | 1.7 |
| Jacobs et al. [31] | NC | Non-ath | 27.0 | 16 | 1.00 | 2 | 6 | HIT | 100 %Pmax | 8 | 12 | 60 | 60 | 0.8 | 8.9 | 3.6 |
| Sharp et al. [32] | NC | Non-ath | 25.5 | 8 | 1.00 | 8 | 32 | HIT | All-out | 8 | 8 | 256 | 30 | 0.13 | 8.3 | 3.3 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | 8 | 24 | HIT | All-out | 3 | 6 | 108 | 30 | 0.17 | 8.2 | 1.5 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | 8 | – | CON | – | – | – | – | – | – | 4.1 | 1.4 |
| Harmer et al. [34] | C | Non-ath | 24.0 | 7 | 0.57 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 8.2 | 3.5 |
| Harmer et al. [34] | C | Non-ath | 25.0 | 8 | 0.63 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 2.4 | 3.3 |
| Shepherd et al. [35] | NC | Non-ath | 22.0 | 8 | 1.00 | 6 | 18 | HIT | All-out | 4 | 6 | 90 | 30 | 0.11 | 7.6 | 3.7 |
| Shepherd et al. [35] | NC | Non-ath | 21.0 | 8 | 1.00 | 6 | 30 | END | 65 %VO2max | – | – | – | 3,000 | – | 15.6 | 3.7 |
| Burgomaster et al. [36] | NC | Non-ath | 24.0 | 10 | 0.50 | 6 | 18 | HIT | All-out | 4 | 6 | 90 | 30 | 0.11 | 7.3 | 2.9 |
| Burgomaster et al. [36] | NC | Non-ath | 23.0 | 10 | 0.50 | 6 | 30 | END | 65 %VO2max | – | – | – | 3,000 | – | 9.8 | 2.9 |
| Bailey et al. [37] | C | Non-ath | 21.0 | 8 | 0.63 | 2 | 6 | HIT | All-out | 4 | 7 | 35 | 30 | 0.13 | 7.1 | 3.3 |
| Bailey et al. [37] | C | Non-ath | 21.0 | 8 | 0.63 | 2 | 6 | END | 90 %GET | – | – | – | 1,200 | – | 0.0 | 3.3 |
| Bailey et al. [37] | C | Non-ath | 21.0 | 8 | 0.63 | 2 | – | CON | – | – | – | – | – | – | −2.1 | 3.3 |
| MacDougall et al. [38] | NC | Non-ath | 22.7 | 12 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.15 | 6.9 | 2.7 |
| Tong et al. [39] | NC | Sed | 22.9 | 8 | 0.25 | 6 | 18 | HIT | 120 %Pmax | 20 | 20 | 360 | 30 | 0.50 | 6.6 | 3.3 |
| Esfarjani and Laursen [40] | NC | Ath | 19.0 | 6 | 1.00 | 10 | 20 | HIT | 130 %vVO2max | 7 | 12 | 190 | 30 | 0.11 | 6.2 | 3.8 |
| Esfarjani and Laursen [40] | NC | Ath | 19.0 | 5 | 1.00 | 10 | 40 | CON | 75 %vVO2max | – | – | – | 3,600 | – | 2.1 | 4.1 |
| Burgomaster et al. [41] | NC | Non-ath | 22.0 | 8 | 1.00 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 5.5 | 3.2 |
| Astorino et al. [42] | C | Non-ath | 25.3 | 20 | 0.55 | 2 | 6 | HIT | All-out | 4 | 6 | 30 | 30 | 0.10 | 5.5 | 2.1 |
| Astorino et al. [42] | C | Non-ath | 22.8 | 9 | 0.55 | 2 | – | CON | – | – | – | – | – | – | 1.6 | 3.1 |
| Sandvei et al. [43] | NC | Non-ath | 25.2 | 11 | 0.36 | 8 | 24 | HIT | All-out | 5 | 10 | 189 | 30 | 0.17 | 5.1 | 2.8 |
| Sandvei et al. [43] | NC | Non-ath | 25.2 | 12 | 0.33 | 8 | 24 | END | 70–80 %HRmax | – | – | – | 2,700 | – | 3.8 | 2.7 |
| Rowan et al. [44] | NC | Ath | 19.5 | 7 | 0.00 | 5 | 10 | HIT | All-out | 5 | 5 | 25 | 30 | 0.13 | 4.7 | 4.0 |
| Rowan et al. [44] | NC | Ath | 19.5 | 6 | 0.00 | 5 | 10 | END | 80 %VO2max | – | – | – | 2,400 | – | 3.3 | 4.3 |
| Stathis et al. [45] | NC | Non-ath | 22.1 | 8 | 0.75 | 7 | 21 | HIT | All-out | 3 | 10 | 153 | 30 | 0.13 | 4.2 | 3.3 |
| Barnes et al. [46] | NC | Ath | 24.9 | 5 | 1.00 | 6 | 12 | HIT | 110 %vVO2max | 8 | 16 | 74 | 40.5 | 0.33 | 3.6 | 4.7 |
| Laursen et al. [47] | C | Ath | 25.0 | 10 | 1.00 | 4 | 8 | HIT | 175 %Pmax | 12 | 12 | 96 | 30 | 0.11 | 3.1 | 2.9 |
| Laursen et al. [47] | C | Ath | 25.0 | 10 | 1.00 | 4 | – | CON | – | – | – | – | – | – | 0.8 | 2.9 |
| Harmer et al. [48] | NC | Non-ath | 22.0 | 7 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.13 | 2.9 | 1.3 |
| Laursen et al. [49] | C | Ath | 23.5 | 7 | 1.00 | 2 | 4 | HIT | 100 %Pmax | 20 | 20 | 80 | 60 | 0.25 | 2.3 | 3.5 |
| Laursen et al. [49] | C | Ath | 23.5 | 7 | 1.00 | 2 | – | CON | – | – | – | – | – | – | −0.8 | 3.5 |
| Dalleck et al. [50] | NC | Non-ath | 21.1 | 10 | 0.45 | 6 | 6 | HIT | 110–120 %Pmax | 6 | 8 | 42 | 30 | 0.14 | −0.7 | 2.9 |
| Dalleck et al. [50] | NC | Non-ath | 21.1 | 10 | 0.45 | 6 | 12 | HIT | 110–120 %Pmax | 6 | 8 | 84 | 30 | 0.14 | −0.6 | 2.9 |
| Iaia et al. [51] | C | Ath | 33.9 | 9 | 1.00 | 4 | 13.6 | HIT | 93 %V max | 8 | 12 | 124 | 30 | 0.17 | −2.4 | 3.1 |
| Iaia et al. [51] | C | Ath | 33.9 | 8 | 1.00 | 4 | 16 | CON | – | – | – | – | 3,138 | – | 0.5 | 3.3 |
Studies are sorted from the largest to the smallest effects on VO2max in the intervention group
Ath athlete, C controlled study, CON control group, END endurance training group, GET gas exchange threshold (determined from a cluster of ventilatory measurements taken during a pre-training incremental test), HIT low-volume high-intensity interval training group, NC non-controlled study, Non-ath non-athlete, Rep repetition, SE standard error, Sed sedentary, VO 2max maximal oxygen uptake, %HR max intensity corresponding to a percentage of maximal heart rate (determined via a pre-training incremental test), %V max intensity corresponding to a percentage of maximum running velocity (determined via a pre-training 30-s all-out sprint run [51]), %VO 2max intensity predicted to elicit a percentage of VO2max (on a treadmill [25] or on a cycle ergometer [26, 36], determined via a pre-training incremental test), %vVO 2max percentage of the running speed predicted to elicit VO2max (determined during a pre-training incremental test [40, 46]), – indicates not applicable, ? indicates data not available
aAll-out: encompasses intensities described by the authors as “maximal” [24]; “near maximal” [43]; “sprints” [33]; “maximum efforts” [38, 44]; “supramaximal” [23]; “sprint training at the highest resistance maintained for 90 rpm” [32]; or “all-out” [21, 22, 25, 28–30, 34–37, 41, 42, 45, 48]. %P max encompasses intensities described as either a ‘percentage of peak watt workload’ [50]; a ‘percentage of the highest 30 s power output completed’ [47]; a ‘percentage of peak work rate’ [20, 39]; a ‘percentage of final completed work rate maintained for 10 s’ [28, 49]; a ‘percentage of peak power output’[31]; a ‘percentage of their final workload’ [27] (all determined via a pre-training incremental test)
Table 2.
Study and subject characteristics for peak power output included in the meta-analysis
| References | Study design | Subjects | Mean age, y | Sample size | Proportion of males | Duration (weeks) | Total sessions | Group | Exercise intensitya | No. of reps | Total reps | Rep duration (s) | Work/rest ratio | Effect on peak power (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Mean | SE | |||||||||||||
| Stathis et al. [45] | NC | Non-ath | 22.1 | 8 | 0.75 | 7 | 21 | HIT | All-out | 3 | 10 | 153 | 30 | 0.13 | 17.1 | 2.9 |
| Burgomaster et al. [52] | NC | Non-ath | 22.0 | 8 | 0.75 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.25 | 15.7 | 2.9 |
| Siahkouhian et al. [21] | NC | Non-ath | 19.1 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 10 | 191 | 30 | 0.13 | 14.4 | 2.4 |
| Forbes et al. [53] | NC | Non-ath | 21.0 | 7 | 0.57 | 2 | 6 | HIT | All-out | 4 | 6 | 30 | 30 | 0.13 | 11.4 | 3.1 |
| MacDougall et al. [38] | NC | Non-ath | 22.7 | 12 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.13 | 10.6 | 2.4 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | All-out | 3 | 4 | 48 | 30 | 0.13 | 10.3 | 2.7 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | 125 %Pmax | 6 | 8 | 96 | 30 | 0.25 | 7.5 | 3.1 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | – | CON | – | – | – | – | – | – | −0.4 | 3.0 |
| Harmer et al. [48] | NC | Non-ath | 22.0 | 7 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 9.4 | 3.1 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | 8 | 24 | HIT | All-out | 3 | 6 | 108 | 30 | 0.17 | 7.7 | 2.9 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | 8 | – | CON | – | – | – | – | – | – | 8.9 | 2.0 |
| Siahkouhian et al. [21] | NC | Ath | 19.4 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 10 | 191 | 30 | 0.13 | 7.9 | 2.4 |
| Richards et al. [54] | NC | Non-ath | 29.0 | 12 | 0.42 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 6.9 | 2.4 |
| McKenna et al. [24] | NC | Non-ath | 20.9 | 8 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 5.8 | 2.9 |
| Esbjörnsson Liljedahl et al. [55] | NC | Non-ath | 25.0 | 10 | 0.00 | 4 | 12 | HIT | All-out | 3 | 3 | 27 | 30 | 0.03 | 5.6 | 2.6 |
| Richards et al. [54] | NC | Non-ath | 25.0 | 11 | 0.27 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 5.5 | 2.5 |
| Burgomaster et al. [41] | NC | Non-ath | 21.0 | 8 | 1.00 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 5.4 | 2.1 |
| Whyte et al. [29] | NC | Sed | 32.1 | 10 | 1.00 | 2 | 6 | HIT | All-out | 4 | 6 | 26 | 30 | 0.11 | 4.7 | 3.3 |
| Allemeier et al. [23] | C | Non-ath | 22.7 | 11 | 1.00 | 6 | 15 | HIT | All-out | 3 | 3 | 45 | 30 | 0.03 | 3.0 | 2.5 |
| Allemeier et al. [23] | C | Non-ath | 24.0 | 6 | 1.00 | 6 | – | CON | – | – | – | – | – | – | 3.0 | 3.3 |
| Jansson et al. [56] | NC | Non-ath | 27.0 | 7 | 1.00 | 4 | 10 | HIT | All-out | 3 | 3 | 30 | 30 | 0.03 | 2.4 | 3.1 |
| McKenna et al. [57] | NC | Non-ath | 18.8 | 6 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.13 | 2.3 | 3.3 |
| Esbjörnsson Liljedahl et al. [55] | NC | Non-ath | 26.0 | 6 | 1.00 | 4 | 12 | HIT | All-out | 3 | 3 | 27 | 30 | 0.03 | 1.4 | 3.3 |
Studies are sorted from the largest to the smallest effects (intervention group) on peak power output
Ath athlete, C controlled study, CON control group, HIT low-volume high-intensity interval training group, NC non-controlled study, Non-ath non-athlete, Rep repetition, SE standard error, Sed sedentary, %P max percentage of the final completed work rate maintained for 10 s during a pre-training incremental test [28], – indicates not applicable
aAll-out: encompasses intensities described as either “maximal” [24, 53, 55, 57]; “sprints” [33]; “maximum efforts” [38]; “supramaximal” [23]; and “all-out” [21, 28, 29, 41, 45, 48, 52, 54, 56]
Table 3.
Study and subject characteristics for mean power output included in the meta-analysis
| References | Study design | Subjects | Mean age, y | Sample size | Proportion of males | Duration (weeks) | Total sessions | Treatment group | Exercise intensitya | No. of reps | Total reps | Rep duration (s) | Work/rest ratio | Effect on mean power (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Mean | SE | |||||||||||||
| Siahkouhian et al. [21] | NC | Non-ath | 19.1 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 10 | 191 | 30 | 0.13 | 17.5 | 3.1 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | All-out | 3 | 4 | 48 | 30 | 0.13 | 17.2 | 6.1 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | 4 | 12 | HIT | 125 %Pmax | 6 | 8 | 96 | 30 | 0.25 | 11.2 | 6.7 |
| Bayati et al. [28] | C | Non-ath | 25.0 | 8 | 1.00 | – | – | CON | – | – | – | – | – | – | 0.3 | 4.4 |
| Siahkouhian et al. [21] | NC | Ath | 19.4 | 12 | 1.00 | 8 | 24 | HIT | All-out | 6 | 10 | 191 | 30 | 0.13 | 11.1 | 3.1 |
| Stathis et al. [45] | NC | Non-ath | 22.1 | 8 | 0.75 | 7 | 21 | HIT | All-out | 3 | 10 | 153 | 30 | 0.13 | 10.7 | 3.8 |
| Esbjörnsson Liljedahl et al. [55] | NC | Non-ath | 25.0 | 10 | 0.00 | 4 | 12 | HIT | All-out | 3 | 3 | 27 | 30 | 0.03 | 10.1 | 2.9 |
| Burgomaster et al. [41] | NC | Non-ath | 21.0 | 8 | 1.00 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 8.7 | 2.9 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | 8 | 24 | HIT | All-out | 3 | 6 | 108 | 30 | 0.17 | 7.1 | 1.6 |
| Barnett et al. [33] | C | Non-ath | 21.2 | 8 | 1.00 | – | – | CON | – | – | – | – | – | – | 2.8 | 1.0 |
| McKenna et al. [24] | NC | Non-ath | 20.9 | 8 | 1.00 | 7 | 21 | HIT | All-out | 4 | 10 | 174 | 30 | 0.14 | 6.1 | 3.8 |
| Allemeier et al. [23] | C | Non-ath | 22.7 | 11 | 1.00 | 6 | 15 | HIT | All-out | 3 | 3 | 45 | 30 | 0.03 | 5.0 | 3.2 |
| Allemeier et al. [23] | C | Non-ath | 24.0 | 6 | 1.00 | 6 | – | CON | – | – | – | – | – | – | 5.0 | 4.4 |
| Forbes et al. [53] | NC | Non-ath | 21.0 | 7 | 0.57 | 2 | 6 | HIT | All-out | 4 | 6 | 30 | 30 | 0.13 | 4.5 | 1.3 |
| Richards et al. [54] | NC | Non-ath | 25.0 | 11 | 0.27 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 3.9 | 3.2 |
| Whyte et al. [29] | NC | Sed | 32.1 | 10 | 1.00 | 2 | 6 | HIT | All-out | 4 | 6 | 26 | 30 | 0.11 | 3.6 | 1.5 |
| Jansson et al. [56] | NC | Non-ath | 27.0 | 7 | 1.00 | 4 | 10 | HIT | All-out | 3 | 3 | 30 | 30 | 0.03 | 2.8 | 4.1 |
| Esbjörnsson Liljedahl et al. [55] | NC | Non-ath | 26.0 | 6 | 1.00 | 4 | 12 | HIT | All-out | 3 | 3 | 27 | 30 | 0.03 | 1.4 | 3.7 |
| Richards et al. [54] | NC | Non-ath | 29.0 | 12 | 0.42 | 2 | 6 | HIT | All-out | 4 | 7 | 30 | 30 | 0.13 | 1.1 | 3.1 |
Studies are sorted from the largest to the smallest effects (intervention group) on mean power output
Ath athlete, C controlled study, CON control group, HIT low-volume high-intensity interval training group, NC non-controlled study, Non-ath non-athlete, Rep repetition, SE standard error, Sed sedentary, %P max percentage of the final completed work rate maintained for 10 s during a pre-training incremental test [28], – indicates not applicable
aAll-out: encompasses intensities described as either “maximal” [24, 53, 55]; “sprints” [33]; “supramaximal” [23]; and “all-out” [21, 28, 29, 41, 45, 54, 56]
Publication Bias and Outliers
To investigate the extent of publication bias, we examined the standard error against the t value for each predicted effect for each outcome, and inspected the plot for signs of asymmetrical scatter [12]. Such a plot is an improved version of the funnel plot, as the scatter of the effects is adjusted for any uncertainty in the estimates and for the contribution of study covariates. Examination of these plots revealed no evidence of the asymmetrical scatter associated with publication bias.
Meta-Analytic Model
The general linear mixed-model procedure (Proc Mixed) in the Statistical Analysis System (Version 9.2, SAS Institute, Cary, NC, USA) was used to perform the meta-analysis. Fixed effects in the model included type of study (controlled, uncontrolled), study-level subject characteristics (sex, training status, baseline VO2max, peak and mean sprint power) and training parameters (number of training sessions, repetition duration, work/rest ratio). We determined the predicted effect of reference training conditions on VO2max, peak and mean sprint power using mean values of baseline fitness, number of sessions and work/rest ratio from all eligible studies. Performance effects were then calculated as the predicted effect under these reference training conditions. The modifying effects of predictors were also calculated, either as differences between levels of a nominal covariate (i.e. male/female, non-athletic/sedentary) or as the effect of approximately two standard deviations (SDs) of a numeric covariate (i.e. a typically high value minus a typically low value) [12]. Random effects in the model were the usual between-subject random effects and a novel within-study random effect to account for within-study repeated measurements (a control treatment and/or more than one training treatment). The residual was set to unity to properly weight the estimates by the inverse of the square or their standard errors. Unexplained true variation within and between studies was estimated by combining the variances for the random effects and was expressed as an SD. The SD was doubled before interpreting its magnitude with the scale used to interpret fixed effects [19], for the same reason that the magnitude of the effect of a linear covariate is evaluated with two SDs of the covariate [12].
Outcome Statistics
We expressed the uncertainty in the estimates of effects on fitness as 90 % confidence limits (CL) and as probabilities that the true value of the meta-analysed effect was trivial, beneficial or harmful in relation to threshold values for benefit and harm. Probabilities were then used to make a qualitative probabilistic inference about the effect [12]. Given that improved aerobic functioning and power output have clinical application [3–7, 15, 16], main treatment effects were considered unclear if the chance of benefit (improved fitness) was high enough to warrant use of the intervention but with an unacceptable risk of harm (reduced fitness). An odds ratio of benefit to harm of <66 was used to identify such unclear effects. This ratio corresponds to a borderline possibly beneficial effect (25 % chance of benefit) and a borderline most unlikely harmful effect (0.5 % risk of harm). All other effects were deemed clinically clear and inference made via estimation of the probability that the true magnitude of the effect was at least as large as our pre-specified thresholds. In the absence of robust anchors for the smallest worthwhile clinical and practical effect on VO2max and sprint power, our inferences were based on standardized thresholds for small, moderate and large changes of 0.2, 0.6 and 1.2 SDs, respectively [12], and derived by averaging appropriate between-subject variances for baseline VO2max, peak and mean sprint power. For VO2max, magnitude thresholds were 3.2, 9.6 and 19.2 % for sedentary subjects, 1.4, 4.1 and 8.1 % for active non-athletic subjects, and 1.4, 4.2 and 8.4 % for athletic subjects. For peak and mean sprint power, thresholds were 1.7, 5.1 and 10.3 and 1.7, 5.2 and 10.5 %, respectively, for male subjects. The chance of the true effect being trivial, beneficial or harmful was interpreted using the following scale: <0.5 % most unlikely; 0.5–5 % very unlikely; 5–25 % unlikely; 25–75 % possibly; 75–95 % likely; 95–99.5 % very likely; >99.5 % most likely [12]. Modifying effects were evaluated non-clinically and deemed unclear if the 90 % CL overlapped the thresholds for the smallest worthwhile positive and negative effects [12].
Results
Maximum Oxygen Consumption
The meta-analysed effects on VO2max of an average low-volume HIT protocol in a controlled trial are shown in Table 4. When compared with control, moderate improvements in VO2max were likely for active non-athletic males and possible for sedentary males and active non-athletic females. A small improvement in VO2max was likely for sedentary females. The effect on athletic males was unclear. With the exception of a possible moderate additional increase in VO2max for subjects with a lower baseline value, the effects of all modifiers were unclear. The comparison of HIT with endurance training was unclear (−1.6 %; 90 % CL ±4.3 %). Unexplained variation expressed as a between-study SD was 2.0 % (±2.7).
Table 4.
Effects of low-volume high-intensity interval training on maximum oxygen consumption following reference training, with modifying effects for study characteristics, subject characteristics and training parameters
| Effect on VO2max (%) | Inference | ||
|---|---|---|---|
| Mean | ±90 % CL | ||
| Effect on treatment groupsa | |||
| Sedentary males | 10.0 | ±5.1 | Possibly moderate ↑ |
| Sedentary females | 7.3 | ±4.8 | Likely small ↑ |
| Active non-athletic males | 6.2 | ±3.1 | Likely moderate ↑ |
| Active non-athletic females | 3.6 | ±4.3 | Possibly moderate ↑ |
| Athletic males | 2.7 | ±4.6 | Unclear |
| Controls | 1.2 | ±2.0 | Unclear |
| Modifying effects | |||
| Baseline VO2max lower by 10 mL·kg−1·min−1 | 3.8 | ±2.5 | Possibly moderate ↑ |
| Athlete vs. active non-athlete | 2.4 | ±5.7 | Unclear |
| Threefold increase in work/rest ratio | −0.3 | ±2.0 | Unclear |
| Threefold increase in no. of sessions | −0.3 | ±2.0 | Unclear |
| Uncontrolled study | −0.7 | ±2.3 | Unclear |
| Sedentary vs. active non-athlete | −2.2 | ±5.7 | Unclear |
| Females | −2.5 | ±4.1 | Unclear |
| Replacement of training (male athletes only) | −2.9 | ±5.3 | Unclear |
Effects on treatment groups are presented as intervention minus control
Reference training: a controlled study of 13 low-volume HIT sessions with a work/rest ratio of 0.16 (0.14 for sedentary females)
CL confidence limit, HIT low-volume high-intensity interval training, VO 2max maximal oxygen uptake, ↑ indicates increase
aActive non-athletic males: baseline VO2max adjusted to 45 mL·kg−1·min−1. Sedentary males: baseline VO2max adjusted to 30 mL·kg−1·min−1. Active non-athletic females: baseline VO2max adjusted to 45 mL·kg−1·min−1. Sedentary females: baseline VO2max adjusted to 30 mL·kg−1·min−1. Athletic males: baseline VO2max adjusted to 60 mL·kg−1·min−1
Sprint Power
The meta-analysed effects of low-volume HIT on 30-s Wingate peak and mean sprint power in a controlled trial are shown in Tables 5 and 6, respectively. With the exception of a possibly moderate improvement in the peak sprint power of controls, all mean effects on sprint power were unclear. There were possibly moderate and likely small improvements in mean and peak sprint power, respectively, following a threefold increase in the number of training sessions. A moderately beneficial improvement in peak sprint power with a greater work/rest ratio was possible and a small additional increase in mean sprint power was possible for subjects with a lower baseline value. All other modifying effects were unclear. Unexplained variation between studies was 2.4 (±2.5) and 1.0 (±2.9) % for peak and mean sprint power, respectively.
Table 5.
Effects of low-volume high-intensity interval training on 30-s Wingate peak sprint power following reference training, with modifying effects for study characteristics, subject characteristics and training parameters
| Effect on peak power (%) | Inference | ||
|---|---|---|---|
| Mean | ±90 % CL | ||
| Effect on treatment groupsa | |||
| Males | 1.8 | ±5.0 | Unclear |
| Controls | 4.5 | ±3.8 | Possibly moderate ↑ |
| Modifying effects | |||
| Fivefold increase in work/rest ratio | 5.7 | ±3.5 | Possibly moderate ↑ |
| Threefold increase in sessions | 2.9 | ±3.5 | Likely small ↑ |
| Females | 2.0 | ±6.3 | Unclear |
| Baseline peak power lower by 5 W/kg | 1.6 | ±3.2 | Unclear |
| Uncontrolled study | −1.0 | ±3.7 | Unclear |
Effects on treatment groups are presented as intervention minus control
Reference training: a controlled study of 12 low-volume HIT sessions with a work/rest ratio of 0.10
CL confidence limit, HIT low-volume high-intensity interval training, ↑ indicates increase
aMales, with baseline peak power output adjusted to 11.5 W/kg
Table 6.
Effects of low-volume high-intensity interval training on 30-s Wingate mean sprint power following reference training, with modifying effects for study characteristics, subject characteristics and training parameters
| Effect on mean power (%) | Inference | ||
|---|---|---|---|
| Mean | ±90 % CL | ||
| Effect on treatment groupsa | |||
| Males | 2.2 | ±10.3 | Unclear |
| Controls | 2.8 | ±8.2 | Unclear |
| Modifying effects | |||
| Threefold increase in sessions | 6.2 | ±3.9 | Possibly moderate ↑ |
| Baseline mean power lower by 4 W/kg | 2.3 | ±3.7 | Possibly small ↑ |
| Fivefold increase in work/rest ratio | 1.5 | ±3.7 | Unclear |
| Females | −0.1 | ±6.9 | Unclear |
| Uncontrolled study | −2.3 | ±4.3 | Unclear |
Effects on treatment groups are presented as intervention minus control
Reference training: a controlled study of 14 low-volume HIT sessions with a work/rest ratio of 0.09
CL confidence limit, HIT low-volume high-intensity interval training, ↑ indicates increase
aMales, with baseline mean power output adjusted to 7.6 W/kg
Discussion
In the previous meta-analyses of Sloth et al. [10] and Gist et al. [11], low-volume HIT improved aerobic fitness and Wingate sprint power, but the effects on different subject groups and other modifying effects were either not analysed or not presented. Our meta-analysis broadens the scope of these previous reviews, as it is the first to include study and subject characteristics in the analysis. Our data revealed HIT to have an apparent adaptive effect on VO2max that favours the less fit. Despite HIT effectively representing repeated Wingate tests, there was no clear effect on measures of performance in the test.
We found that a mean protocol of 13 HIT sessions with a work/rest ratio of 0.16 led to moderate improvements in the VO2max of sedentary and non-athletic males and females. This main finding is consistent with the recent work of Sloth et al. [10], Gist et al. [11] and Bacon et al. [7], who reported standardized moderate effects on VO2max for HIT and high-intensity aerobic interval training, respectively. A combination of central and peripheral adaptations promoting an enhanced availability, extraction and utilization of oxygen may explain such improvements following intensive interval-training protocols. However, mechanisms responsible for increased VO2max following HIT were not the focus of our review. Comprehensive reviews of the possible underlying mechanisms are available elsewhere [9, 10].
Gibala et al. [58] reported low-volume HIT to be a time-efficient strategy for rapid physiological and performance improvements that are comparable to improvements following traditional endurance training. The random effects component of our mixed model enabled us to include studies where the reference group was traditional endurance training rather than no training. Here, the comparison between the two types of training was unclear. This finding is consistent with that of Gist et al. [11], who reported a trivial effect of HIT on VO2max when compared with endurance training controls. More studies are therefore required to examine the effectiveness of HIT versus traditional endurance training for training-induced endurance gains. The effect of HIT on VO2max was greater for the less fit, which is consistent with training in general having greater effects on the less fit [59]. For already highly trained athletes who replaced their usual training with HIT, as opposed to adding the HIT, the effect on VO2max was unclear. This finding also indicates the need for more research, providing that elite athletes can be convinced to experiment with their normal training programmes [9]. Despite reporting no analytical data for the potentially modifying effect of training duration, Sloth et al. [10] and Gist et al. [11] reported no clear effects of the length of HIT intervention on the magnitude of VO2max improvement. The data presented in our more extensive meta-analysis have still not resolved this issue.
On the basis of the CLs, low-volume HIT had an unclear effect on peak and mean sprint power that could at most be a moderate beneficial or a small harmful effect. These results contrast with those of Sloth et al. [10], who reported enhanced peak and mean power following HIT. Their assertion was based on nine studies [24, 28–30, 33, 36, 41, 42, 52], without a meta-analysis of the mean effect and its uncertainty. Three of these studies [30, 36, 42] were excluded from our analysis, owing to difficulties in obtaining precise baseline and post-intervention data during the data extraction process. An enhanced sprint power following HIT was expected, given that all-out training increases enzymatic activity related to anaerobic metabolism [13]. Furthermore, studies showing strong similarities between testing and training routines are more likely to show training improvements [60]. However, when measured relative to controls, the meta-analysed effect of HIT on sprint power was unclear. Improvements of 4.5 % in peak sprint power and 2.8 % in mean sprint power of control subjects may have represented a learning effect on the Wingate test or provide some evidence of compensatory rivalry (e.g. greater effort by controls). There was some evidence of a dose–response relationship and a greater effect for the less fit. The finding of a possibly moderate enhancement in peak sprint power with a fivefold increase in repetition work/rest ratio could be explained by greater phosphocreatine resynthesis in the recovery phase [61, 62].
There was considerable uncertainty in the SDs representing the residual between-study variation in the mean effect of the treatment on the three measures of fitness, but in this sample of studies the observed magnitudes (after doubling the SDs) were small to moderate, depending on the measure of performance and the subject group. This SD needs to be added to and subtracted from the main effect to evaluate the magnitude of the HIT treatment in a specific setting. For example, the mean effect of HIT on VO2max for active non-athletic males (6.2 %; moderate) in any given setting could be anywhere from 4.2 % (very likely small) to 8.2 % (possibly large). Such differences between the effects of training in the different studies presumably reflect differences in subject characteristics and training protocols that are not properly accounted for by the published data. Some data may also have been analysed or reported erroneously.
We propose several areas for future research, along with suggestions for those publishing research in this area. Given that the age of participants included within our meta-analysis was mainly young adults, it is evident that research is required to clarify the effects of low-volume HIT in older populations. Moderating effects of changing the exercise dose on VO2max were unclear, as was the replacement of athletes’ usual aerobic training with HIT, indicating that more research is necessary to investigate these predictors. However, we do recommend that modifying effects are interpreted with slight caution, as when a covariate is a subject characteristic averaged over study subjects, the observed meta-regression relationships might not hold at the individual study level [63]. The practicality of low-volume HIT warrants further investigation, given that repeated bouts of maximal exercise require high levels of motivation [9]. Adherence to unsupervised training also needs investigation [29]. We concur with the need to test the effectiveness of low-volume HIT via large-scale, multi-centre, randomized clinical trials in various clinical populations and on long-term clinical outcome measures [64]. Of further benefit would be the reporting of full inferential statistics, such as SD of change scores or exact p values in training and control groups, to enable meta-analysis of the magnitude of individual responses. Finally, the findings of a training study are of very little or no value without precise information of the training itself [65]. We therefore encourage authors to report physiological responses during HIT sessions, as this practice will help to demonstrate that the fidelity of an intervention has been upheld for all subjects.
Conclusions
Low-volume HIT is increasingly being used for aerobic adaptations previously achieved with traditional endurance training. Our meta-analysis provides evidence of substantial improvements in the endurance fitness of sedentary and non-athletic subjects following repeated bouts of brief maximal intermittent exercise. The effect of HIT on sprint power should be determined with more studies.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
References
- 1.Helgerud J, Høydal K, Wang E, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 2.Buchheit M, Laursen P. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013;43:313–338. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 3.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Guiraud T, Nigam A, Gremeaux V, et al. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42(7):587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Hwang CL, Wu YT, Chou CH. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev. 2011;31(6):378–385. doi: 10.1097/HCR.0b013e31822f16cb. [DOI] [PubMed] [Google Scholar]
- 6.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2013. doi:10.1136/bjsports-2013-092576. [DOI] [PubMed]
- 7.Bacon AP, Carter RE, Ogle EA, et al. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013;8(9):e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013;43:927–954. doi: 10.1007/s40279-013-0066-5. [DOI] [PubMed] [Google Scholar]
- 9.Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloth M, Sloth D, Overgaard K, et al. Effects of sprint interval training on VO2max and aerobic exercise performance: a systematic review and meta-analysis. Scand J Med Sci Sports. 2013. doi:10.1111/sms.12092. [DOI] [PubMed]
- 11.Gist NH, Fedewa MV, Dishman RK, et al. Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med. 2014;44(2):269–279. doi: 10.1007/s40279-013-0115-0. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–12. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 13.Rodas G, Ventura JL, Cadefau JA, et al. A short training programme for the rapid improvement of both aerobic and anaerobic metabolism. Eur J Appl Physiol. 2000;82(5–6):480–486. doi: 10.1007/s004210000223. [DOI] [PubMed] [Google Scholar]
- 14.Davison RR, Van Someren KA, Jones AM. Physiological monitoring of the Olympic athlete. J Sports Sci. 2009;27(13):1433–1442. doi: 10.1080/02640410903045337. [DOI] [PubMed] [Google Scholar]
- 15.Katula JA, Rejeski WJ, Marsh AP. Enhancing quality of life in older adults: a comparison of muscular strength and power training. Health Qual Life Outcomes. 2008;6:45. doi: 10.1186/1477-7525-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruda KV, Hicks AL, McCartney N. Training for muscle power in older adults: effects on functional abilities. Can J Appl Physiol. 2003;28(2):178–189. doi: 10.1139/h03-014. [DOI] [PubMed] [Google Scholar]
- 17.Vandenbogaerde TJ, Hopkins WG. Effects of acute carbohydrate supplementation on endurance performance: a meta-analysis. Sports Med. 2011;41(9):773–792. doi: 10.2165/11590520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med. 2011;41(10):801–814. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Smith TB, Hopkins WG. Variability and predictability of finals times of elite rowers. Med Sci Sports Exerc. 2011;43:2155–2160. doi: 10.1249/MSS.0b013e31821d3f8e. [DOI] [PubMed] [Google Scholar]
- 20.Rakobowchuk M, Harris E, Taylor A, et al. Heavy and moderate interval exercise training alters low-flow-mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol. 2012;97(3):375–385. doi: 10.1113/expphysiol.2011.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siahkouhian M, Khodadadi D, Shahmoradi K. Effects of high-intensity interval training on aerobic and anaerobic indices: comparison of physical active and inactive men. Sci Sports. 2013;28:e119–e125. doi: 10.1016/j.scispo.2012.11.006. [DOI] [Google Scholar]
- 22.Trilk JL, Singhal A, Bigelman KA, et al. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur J Appl Physiol. 2011;111:1591–1597. doi: 10.1007/s00421-010-1777-z. [DOI] [PubMed] [Google Scholar]
- 23.Allemeier CA, Fry AC, Johnson P, et al. Effects of sprint cycle training on human skeletal muscle. J Appl Physiol. 1994;77(5):2385–2390. doi: 10.1152/jappl.1994.77.5.2385. [DOI] [PubMed] [Google Scholar]
- 24.McKenna MJ, Heigenhauser GJ, McKelvie RS, et al. Enhanced pulmonary and active skeletal muscle gas exchange during intense exercise after sprint training in men. J Physiol. 1997;501(Pt 3):703–716. doi: 10.1111/j.1469-7793.1997.703bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macpherson RE, Hazell TJ, Olver TD, et al. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc. 2011;43:115–122. doi: 10.1249/MSS.0b013e3181e5eacd. [DOI] [PubMed] [Google Scholar]
- 26.Esfandiari S, Sasson Z, Goodman JM. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur J Appl Physiol. 2014;114(2):331–343. doi: 10.1007/s00421-013-2773-x. [DOI] [PubMed] [Google Scholar]
- 27.Dunham C, Harms CA. Effects of high-intensity interval training on pulmonary function. Eur J Appl Physiol. 2012;112:3061–3068. doi: 10.1007/s00421-011-2285-5. [DOI] [PubMed] [Google Scholar]
- 28.Bayati M, Farzad B, Gharakhanlou R, et al. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble “all-out” sprint interval training. J Sports Sci Med. 2011;10:571–576. [PMC free article] [PubMed] [Google Scholar]
- 29.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59:1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Hazell TJ, Macpherson RE, Gravelle BM, et al. 10 or 30-s sprint interval training bouts enhance both aerobic and anaerobic performance. Eur J Appl Physiol. 2010;110:153–160. doi: 10.1007/s00421-010-1474-y. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs RA, Flück D, Bonne TC, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol. 2013;115:785–793. doi: 10.1152/japplphysiol.00445.2013. [DOI] [PubMed] [Google Scholar]
- 32.Sharp RL, Costill DL, Fink WJ, et al. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int J Sports Med. 1986;7(1):13–17. doi: 10.1055/s-2008-1025727. [DOI] [PubMed] [Google Scholar]
- 33.Barnett C, Carey M, Proietto J, et al. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004;7:314–322. doi: 10.1016/S1440-2440(04)80026-4. [DOI] [PubMed] [Google Scholar]
- 34.Harmer AR, Ruell PA, McKenna MJ, et al. Effects of sprint training on extrarenal potassium regulation with intense exercise in Type 1 diabetes. J Appl Physiol. 2006;100(1):26–34. doi: 10.1152/japplphysiol.00240.2005. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd SO, Cocks M, Tipton KD, et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol. 2013;591(3):657–675. doi: 10.1113/jphysiol.2012.240952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgomaster KA, Howarth KR, Phillips SM, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey SJ, Wilkerson DP, Dimenna FJ, et al. Influence of repeated sprint training on pulmonary O2 uptake and muscle deoxygenation kinetics in humans. J Appl Physiol. 2009;106:1875–1887. doi: 10.1152/japplphysiol.00144.2009. [DOI] [PubMed] [Google Scholar]
- 38.MacDougall JD, Hicks AL, MacDonald JR, et al. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol. 1998;84:2138–2142. doi: 10.1063/1.368275. [DOI] [PubMed] [Google Scholar]
- 39.Tong TK, Chung PK, Leung RW, et al. Effects of non-Wingate-based high-intensity interval training on cardiorespiratory fitness and aerobic-based exercise capacity in sedentary subjects: a preliminary study. J Exerc Sci Fit. 2011;9(2):75–81. doi: 10.1016/S1728-869X(12)60001-X. [DOI] [Google Scholar]
- 40.Esfarjani F, Laursen PB. Manipulating high-intensity interval training: effects on VO2max, the lactate threshold and 3000 m running performance in moderately trained males. J Sci Med Sport. 2007;10(1):27–35. doi: 10.1016/j.jsams.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol. 2006;100:2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 42.Astorino TA, Allen RP, Roberson DW, et al. Effect of high-intensity interval training on cardiovascular function, VO2max, and muscular force. J Strength Cond Res. 2012;26:138–145. doi: 10.1519/JSC.0b013e318218dd77. [DOI] [PubMed] [Google Scholar]
- 43.Sandvei M, Jeppesen PB, Støen L, et al. Sprint interval running increases insulin sensitivity in young healthy subjects. Arch Physiol Biochem. 2012;118(3):139–147. doi: 10.3109/13813455.2012.677454. [DOI] [PubMed] [Google Scholar]
- 44.Rowan AE, Kueffner TE, Stavrianeas S. Short duration high-intensity interval training improves aerobic conditioning of female college soccer players. Int J Exerc Sci. 2012;5(3):232–238. [Google Scholar]
- 45.Stathis CG, Febbraio MA, Carey MF, et al. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol. 1994;76(4):1802–1809. doi: 10.1152/jappl.1994.76.4.1802. [DOI] [PubMed] [Google Scholar]
- 46.Barnes KR, Hopkins WG, McGuigan MR, et al. Effects of different uphill interval-training programs on running economy and performance. Int J Sports Physiol Perform. 2013;8:639–647. doi: 10.1123/ijspp.8.6.639. [DOI] [PubMed] [Google Scholar]
- 47.Laursen PB, Shing CM, Peake JM, et al. Interval training program optimization in highly trained endurance cyclists. Med Sci Sports Exerc. 2002;34(11):1801–1807. doi: 10.1097/00005768-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Harmer AR, McKenna MJ, Sutton JR, et al. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol. 2000;89(5):1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- 49.Laursen PB, Blanchard MA, Jenkins DG. Acute high-intensity interval training improves Tvent and peak power output in highly trained males. Can J Appl Physiol. 2002;27(4):336–348. doi: 10.1139/h02-019. [DOI] [PubMed] [Google Scholar]
- 50.Dalleck L, Bushman TT, Crain RD, et al. Dose–response relationship between interval training frequency and magnitude of improvement in lactate threshold. Int J Sports Med. 2010;31(8):567–571. doi: 10.1055/s-0030-1254136. [DOI] [PubMed] [Google Scholar]
- 51.Iaia FM, Hellsten Y, Nielsen JJ, et al. Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J Appl Physiol. 2009;106(1):73–80. doi: 10.1152/japplphysiol.90676.2008. [DOI] [PubMed] [Google Scholar]
- 52.Burgomaster KA, Hughes SC, Heigenhauser GJ, et al. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 53.Forbes SC, Slade JM, Meyer RA. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Physiol Nutr Metab. 2008;33(6):1124–1131. doi: 10.1139/H08-099. [DOI] [PubMed] [Google Scholar]
- 54.Richards JC, Johnson TK, Kuzma JN, et al. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol. 2010;588:2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esbjörnsson Liljedahl M, Holm I, Sylvén C, et al. Different responses of skeletal muscle following sprint training in men and women. Eur J Appl Physiol Occup Physiol. 1996;74(4):375–383. doi: 10.1007/BF02226935. [DOI] [PubMed] [Google Scholar]
- 56.Jansson E, Esbjornsson M, Holm I, et al. Increase in the proportion of fast-twitch muscle fibres by sprint training. Acta Physiol Scand. 1990;140:359–363. doi: 10.1111/j.1748-1716.1990.tb09010.x. [DOI] [PubMed] [Google Scholar]
- 57.McKenna MJ, Schmidt TA, Hargreaves M, et al. Sprint training increases human skeletal muscle Na+–K+-ATPase concentration and improves K+ regulation. J Appl Physiol. 1993;75(1):173–180. doi: 10.1152/jappl.1993.75.1.173. [DOI] [PubMed] [Google Scholar]
- 58.Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilmore JH, Costill DL. Physiology of sport and exercise. 2. USA: Human Kinetics; 1999. [Google Scholar]
- 60.Buchheit M. Should we be recommending repeated sprints to improve repeated-sprint performance? Sports Med. 2012;42(2):169–172. doi: 10.2165/11598230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Bogdanis GC, Nevill ME, Boobis LH, et al. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol. 1996;80:876–884. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 62.Bishop D, Girard O, Mendez-Villanueva A. Repeated-sprint ability—part II: recommendations for training. Sports Med. 2011;41(9):741–756. doi: 10.2165/11590560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Petkova E, Tarpey T, Huang L, et al. Interpreting meta-regression: application to recent controversies in antidepressants’ efficacy. Stat Med. 2013;32(17):2875–2892. doi: 10.1002/sim.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawley JA, Gibala MJ. What’s new since Hippocrates? Preventing type 2 diabetes by physical exercise and diet. Diabetologia. 2012;55(3):535–539. doi: 10.1007/s00125-012-2460-1. [DOI] [PubMed] [Google Scholar]
- 65.Mujika I. The alphabet of sport science research starts with Q. Int J Sports Physiol Perform. 2013;8:465–466. doi: 10.1123/ijspp.8.5.465. [DOI] [PubMed] [Google Scholar]


