Figure 2.
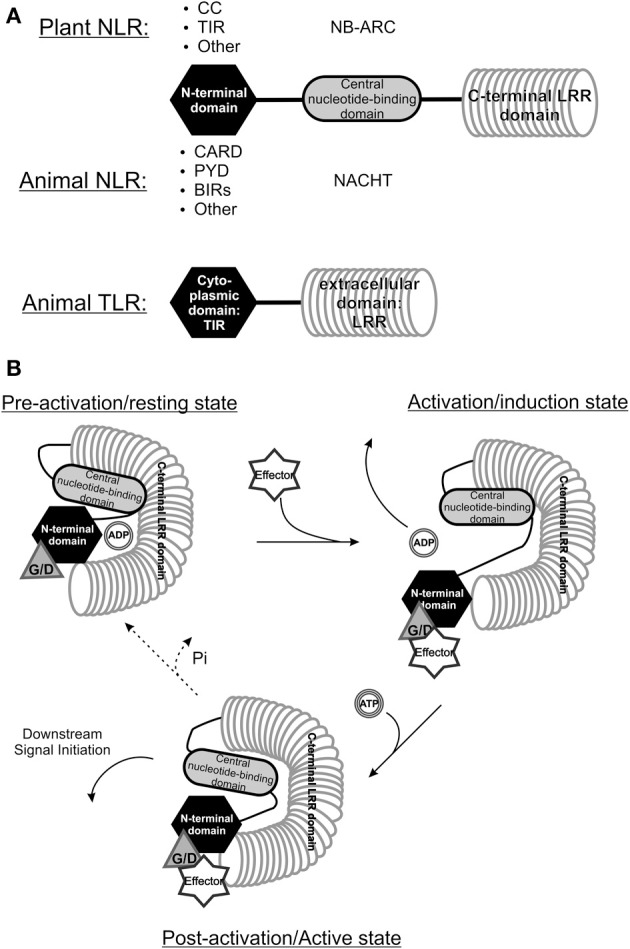
Comparison between the structure of plant and animal NLRs. (A) The structure of “Nucleotide binding and leucine rich repeat proteins” (NLRs) from the animal and plant kingdom share highest homology, as all proteins belonging to this class have a C-terminal leucine rich repeat (LRR), a central nucleotide binding domain and a varying N-terminal domain (modified from Maekawa et al., 2011b). Animal TLRs also contain an (extracellular) LRR domain and possess a TIR-domain, they do however, lack a nucleotide binding domain. CC, Coiled-coil; TIR, Toll-interleukin receptor; CARD, Caspase-activation and recruitment domain; PYR, Pyrin domain; BIR, Baculovirus inhibitor-of-apoptosis repeats; NB-ARC, Nucleotide binding and Apaf1-R protein-CED4 domain; NACHT, NAIP – CIITA - HET-E - TP1 domain. (B) A model of NB-LRR R protein recognizing a specific Avr protein through a guardee or decoy host protein. Upon interaction with the Avr protein the R protein conformationally changes and the ADP can be exchanged for ATP, leading to a second conformational change triggering downstream resistance (Modified from Lukasik and Takken, 2009). Whether the R protein returns to its resting state is not known yet. G/D, Guardee/Decoy.
