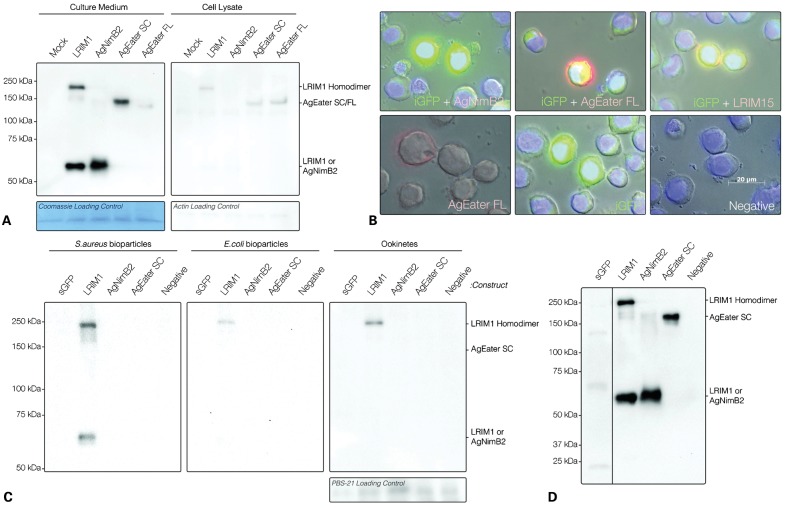
Figure 4.
Localization and binding properties of recombinant AGNimB2 and AgEater proteins. (A) A non-reducing SDS-PAGE western blot (WB) of culture medium and cell lysate from transfected Sf9 cell lines, probed using an anti-Strep tag antibody. LRIM1 is expressed in both its dimeric (150 kDa) and monomeric (59.6 kDa) form, rAgNimB2 is monomeric (44 kDa), rAgEater secreted (SC) (97 kDa) and full-length (FL) (109 kDa) are monomeric. As a control, a mock transfection was conducted without DNA. All proteins are secreted into the culture medium, with only LRIM1 and rAgEater present at detectable levels in the cell lysate. Actin was used as a loading control for cell lysate, and Coomassie stains carried out on all samples. (B) Fluorescent images of Sf9 cells transfected with expression plasmids (pIEX10) containing intracellular GFP (iGFP) alone or together with LRIM15, rAgNimB2, rAgEater FL, or mock transfected (Negative). Cells imaged by phase contrast microscopy and fluorescent images overlaid; green: GFP, Red: Strep tag-labeled proteins (LRIM15, rAgNimB2, and rAgEater FL), Blue: DAPI. (D) Non-reducing SDS-PAGE WB probed for Strep tag containing proteins which were selected for binding ability to either Staphylococcus aureus bioparticles, Escherichia coli bioparticles, or Plasmodium berghei ookinetes. Secreted GFP (sGFP) was used as a negative control, LRIM1 as a positive control. Only LRIM1 showed binding to E. coli, S. aureus, and ookinetes, with only S. aureus binding both the LRIM1 monomer and homodimer. PBS-21 was used as a loading control in the ookinete assay. Even loading for all samples was confirmed by Coomassie staining (not shown). (D) Non-reducing SDS-PAGE WB probed for Strep tag containing recombinant protein to confirm their presence in culture medium from transfected sf9 cells (transfected with sGFP, LRIM1, AgNimB2, and AgEater SC) for use in binding assays. All blots are representative of two independent biological replicates.