Figure 2.
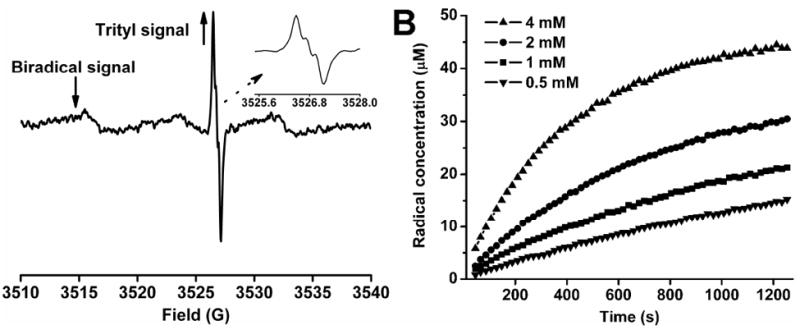
(A) EPR spectrum obtained from the reaction mixture containing TNN14 (50 μM) and ascorbate (4 mM) in PBS (pH 7.4, 50 mM) after 45 s at room temperature; (↑) indicates the increasing signal of the trityl monoradical TNN14-H which resulted from the reduction of the biradical TNN14, (↓) shows the decreasing signal of the biradical TNN14; the inset shows an expanded portion of the spectrum of the trityl monoradical in order to better visualize its triplet hyperfine structure. In order to detect both signals from the biradical and the resulting trityl radical without any distortion, spectra were recorded with 1 mW microwave power and 0.08 G modulation amplitude. These low values of microwave power and modulation amplitude are required to prevent broadening or saturation of the trityl radical spectrum. (B) Plots of the TNN14-H concentration as a function of time at various ascorbate concentrations at room temperature.
