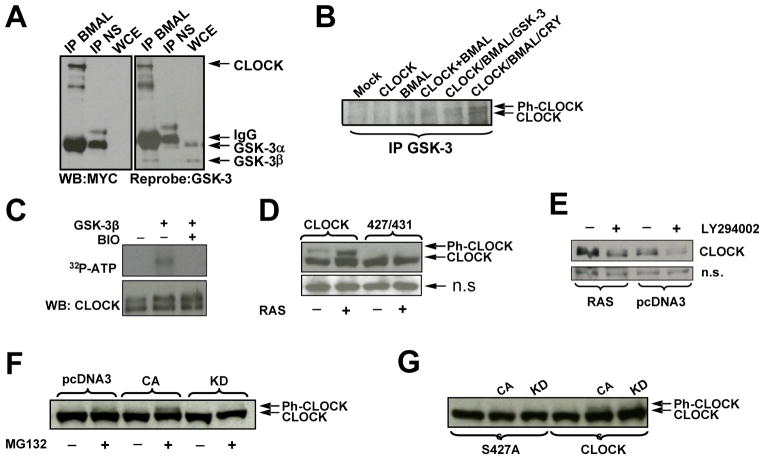
Fig. 4. CLOCK phospho-degron is regulated by the activity of RAS/PI3K/AKT/GSK3 pathway.
A,B GSK-3β is pulled down in complex with CLOCK and BMAL1
A. 293T cells expressing ectopic CLOCK and BMAL1 were immunoprecipitated with anti-BMAL or normal serum (NS) and analyzed for CLOCK by anti-MYC Western Blot; the same blot was re-probed for the detection of GSK-3. The monoclonal antibody against GSK-3 recognized both alpha and beta forms; GSK-3 α migrated with the IgG heavy chain as denoted by arrows.
B. 293T cells expressing various combinations of clock proteins, as indicated, were immunoprecipitated with anti-GSK-3 antibody followed by CLOCK determination by Western Blot using anti-MYC antibody. Arrows indicate the position of phosphorylated (Ph-CLOCK) and un-phosphorylated CLOCK.
C. GSK-3β phosphorylates CLOCK in vitro. Lysate from cells expressing ectopic CLOCK and BMAL were immunoprecipitated with anti-CLOCK antibody in a stringent RIPA buffer. The immunoprecipitate was incubated in kinase buffer in various combinations of recombinant GSK-3β, BIO, and 32P-ATP followed by incubation at 30 C°. After 1 hr, samples were boiled in SDS buffer, resolved by SDS-PAGE and transferred to nitrocellulose for autoradiogram analysis.
D. Inhibition of GSK-3, via constitutive activation of the RAS/AKT pathway, specifically increases phospho-competent CLOCK levels compared to phospho-deficient CLOCK. 293T cells were transfected with plasmids encoding for BMAL1 and either PC-CLOCK or PD-CLOCK S427/431A, and with either constitutively active RAS or empty vector, as indicated. Cell lysates were analyzed by Western Blot using anti-MYC antibody for the detection of CLOCK.
E. Up-regulation of GSK-3, via inhibition of the RAS pathway, destabilizes CLOCK. 293T cells expressing CLOCK, BMAL and RAS or empty vector, pcDNA3 were treated with either DMSO or PI3K/AKT inhibitor, LY294002 as indicated. A non-specific (n.s.) protein is shown as an indicator of loading.
F. GSK-β3 mediates CLOCK phosphorylation in cell culture. Cells expressing ectopic CLOCK and RAS were co-expressed with either active (CA) or inactive (KD) GSK-3β, as indicated. Cells were treated 3.5 hours with MG132 prior to harvest. CLOCK was visualized by anti-MYC Western Blot.
G. GSK-3β fails to phosphorylate phospho-mutant CLOCK S427A. Cell extract were prepared and analyzed as described in Fig. 4F.