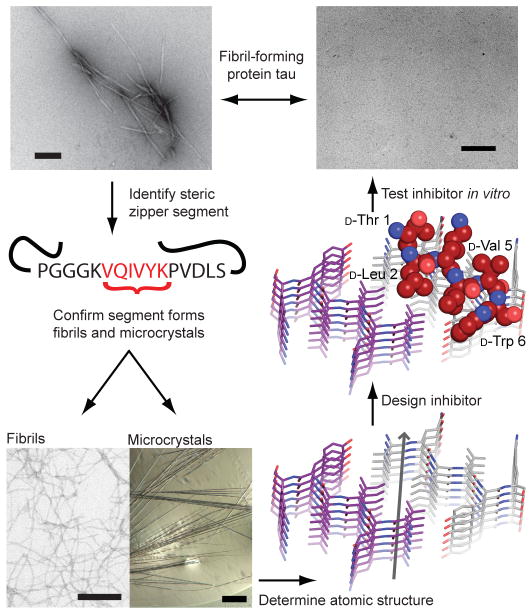
Figure 1. Scheme for the design and characterization of peptide inhibitors of amyloid fibrillation.
Tau constructs form fibers in vitro (top left)24. The VQIVYK segment in isolation forms fibers and microcrystals (bottom left). The atomic structure of the fiber-like VQIVYK segment reveals a characteristic steric zipper motif15, comprising a pair of interacting β-sheets running along the fiber axis (grey arrow), in purple and grey (bottom right). We designed a D-amino acid peptide to bind to the end of the steric zipper template and prevent fiber elongation (middle right). The D-peptide, in red, is designed to satisfy hydrogen bonds and make favorable apolar interactions with the molecule below, while preventing the addition of other molecules above and on the opposite β-sheet. As shown in vitro, the designed D-peptide prevents the formation of fibers when incubated with tau K19 (upper right). Scale bars are 100 μm and 200 nm on the microcrystal image and electron micrographs, respectively.