Abstract
The eye has been one of the most intensively studied organs in Drosophila. The wealth of knowledge about its development, as well as the reagents that have been developed, and the fact that the eye is dispensable for survival, also make the eye suitable for genetic interaction studies and genetic screens. This chapter provides a brief overview of the methods developed to image and probe eye development at multiple developmental stages, including live imaging, immunostaining of fixed tissues, in situ hybridizations, and scanning electron microscopy and color photography of adult eyes. Also summarized are genetic approaches that can be performed in the eye, including mosaic analysis and conditional mutation, gene misexpression and knockdown, and forward genetic and modifier screens.
Keywords: Drosophila eye, live imaging, immunocytochemistry, in situ hybridization, scanning electron microscopy, genetic screen
1. Introduction
Studies of the Drosophila eye have made multiple contributions to developmental biology. The Drosophila eye is one of the first parts of the adult nervous system to differentiate, and the most substantial part of the nervous system to differentiate outside the protection of a pupal case or egg shell. Furthermore, while the eye is required for vision, it is not required for life, facilitating the study of lethal genotypes. The color and detail of eye structure has made the eye appropriate for forward genetic screens.
The Drosophila eye differentiates from an epithelium, the eye imaginal disc, in the late third larva instar and early part of the pupa [1]. As a compound eye, the Drosophila eye contains ~750 ommatidia, or unit eyes, each of which contains eight photoreceptor neurons (Figure 1A). Each ommatidium also contains four non-neuronal cone cells and two primary pigment cells, and is surrounded by a shared lattice of secondary and tertiary pigment cells and interommatidial bristle organs. The ommatidial structure is very precisely repetitive in normal individuals, so that subtle abnormalities may be recognized (Figure 1B).
Figure 1. Summary of Drosophila eye structure and development.
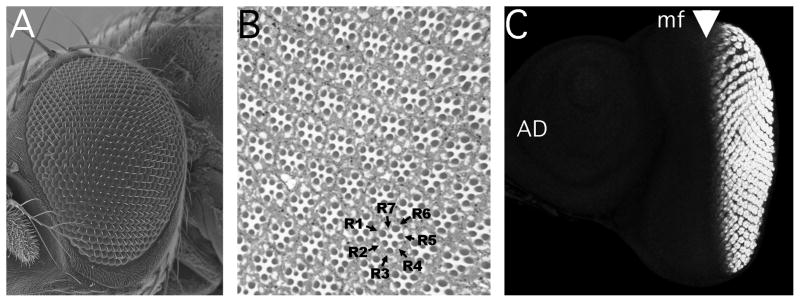
A). Scanning Electron Micrograph of the adult eye, with anterior to the left. Each adult compound eye contains ~750 facets, or ommatidia.
B). Thick section through an eye revealing the repetitive cellular pattern. The rhabdomeres of seven of the eight photoreceptor neurons are apparent in each plane of section, and are labeled for one ommatidium.
C). Eye-antennal imaginal disc from a mid-third instar larvae (~96h after egg laying). Clusters of differnetiating photoreceptor neurons behing the morphogenetic furrow (arrowhead) are labeled with antibody against ELAV. The morphogenetic furrow moves from posterior to anterior (left ot right in this image) across the eye disc. Ahead of the morphogenetic furrow, individual cell fates remain unspecified and no ELAV labeling is seen. The antennal disc (AD) is also unlabeled.
Modern study of the Drosophila eye may be traced to the classic paper of Ready et al [2], which in addition to descriptive study, also demonstrated that ommatidia were not clonal units. Lawrence and Green [3] later demonstrated that almost any pair of eye cell types could be related at the final mitosis, ruling out inheritance of determination states in eye cell fate specification, thereby implying that cell interactions must specify this highly repetitive structure. Electron microscopic reconstruction of ommatidial assembly then led to a model that short range cell interactions determined the majority of eye cell fates [4]. This understanding underscored the molecular genetic studies of eye development that were instrumental for uncovering many aspects of developmental signaling by receptor tyrosine kinases, the Notch pathway, and other universal developmental regulators [5–10].
While many important questions remain in the study of eye development itself, the tools developed in the course of Drosophila eye studies, coupled with the readily apparent structure and dispensable function of the organ, also make the Drosophila eye an exemplary system for investigating general biological processes, and for unbiased genetic interaction screens with the potential to characterize new pathways, such as those associated with human disease genes. The purpose of this chapter is to outline some of the basic tools, both experimental and genetic, that can be used to characterize development and gene function using the Drosophila eye. It is not a list of protocols, nor intended as an update for the expert, but provides summaries of the main approaches that would be routine in many ‘eye labs’, wherever possible including citations to more detailed methods. This may provide an entry point and resource for those considering exploiting Drosophila eye methods for their research.
2 Eye Methods
2.1 Development and Anatomy of the eye
General features of the Drosophila eye and eye-imaginal disc are shown in Figure 1. For more detailed accounts of the development of the eye imaginal disc, see [1].
Cells that will contribute to the Drosophila eye, head capsule, and antenna, separate from the larval epidermis during embryogenesis [11, 12]. After hatching (about 22h after egg laying at 25°C), imaginal discs grow suspended in the body cavity of the three successive larval instars until pupariation (about 120h after egg laying at 25°C). The distinction between antennal and eye portions becomes more apparent over larval life. By the third larval instar (~72h – ~120h after egg laying), the ‘eye disc’ portion also contains cells that will contribute to the adult head epidermis. Specification and differentiation of individual retinal cells begins early in the third larval instar, and continues beyond pupariation. Once all the cells are specified, head eversion moves the eye and head tissues into their adult configuration before the end of pupation. Adults emerge typically from the pupae 9 days after egg laying at 25°C. For a detailed account of the Drosophila lifecycle, see [13].
Specification of the individual retinal cells begins in the third larval instar, ~72h after egg laying, and is associated with a ‘morphogenetic furrow’ that progresses across the eye disc epithelium [1, 2]. The morphogenetic furrow is an indentation of the epithelium, associated with cell shape changes and transient cell cycle arrest. The morphogenetic furrow appears to be one of the first aspects of adult differentiation to begin after the commitment to begin adult differentiation.
Within the morphogenetic furrow, individual R8 photoreceptor precursors are specified that each found an ommatidium by recruiting adjacent cells to other photoreceptor cell fates (Figure 1C) [4, 14]. Many publications describe the cellular sequence of ommatidial assembly in the eye disc [1, 4, 15, 16]. One new ommatidial column begins every 90–120 min (slowing with time), up to a total of ~30 columns. The progression of the morphogenetic furrow across the disc means that each eye disc contains ommatidia at a range of developmental stages, arrayed in sequence posterior to the morphogenetic furrow.
Once specified, cells begin to execute their specific differentiation programs [1]. Still before pupariation, larval photoreceptor neurons extend axons down the optic stalk towards the brain, where the find their target zones in the lamina and medulla. Glial cells migrate in the reverse direction, following photoreceptor axons from the brain back to the eye disc.
When the larva pupariates and pupation begins, the eye imaginal discs at first continues to occupy a peripheral position close to the surface of the animal. Later, tissue movements associated with head eversion rearrange the eye and brain into their adult organization.
2.2 Live Imaging
2.2.1 Live imaging eye imaginal discs
Two approaches have been described to image living eye discs. One is to visualize the eye disc within the immobilized pupa, the other cultures eye discs explanted from the larva. In both methods the eye discs should express a fluorescent-tagged protein for imaging, such as Cadherin::GFP which label cell boundaries [17], or Histone 2N::GFP which labels chromatin [18].
2.2.2 Imaging the developing eye within the pupa
During the first part of pupal development, while the morphogenetic furrow is still advancing, the eye disc lies close to the surface of the animal and can be imaged through the pupal epidermis after removing part of the pupal case. More detailed protocols are available elsewhere [19, 20]. Animals are immobilized at the white prepupa stage on a microscope slide coated with double-sided tape. The operculum region of the puparium is removed and a three-sided supporting enclosure fashioned out of parafilm or similar material. Within this enclosure the exposed pupa is bathed in buffer and covered with a cover-slip. The whole assemblage is sealed with paraffin on three sides with halocarbon oil at the free side to permit gas exchange. Drugs can be included in the buffer, indeed levamisole is normally included to paralyze the animals. Since the animals do not feed during the pupal stage, they can be paralyzed without affecting nutrition, an advantage of imaging at this stage.
2.2.3 Imaging explanted eye discs
For a more detailed protocol see [21]. Eye-antennal discs are removed from later third-instar larvae (see section 2.3.2 below) in PBS and transferred immediately to a glass-bottom culture dishes (eg MatTek Corporation), where they adhere to the glass. The PBS is immediately replaced with Schneider culture medium supplemented with 10% heat-inactivated Fetal Bovine Serum. Eye discs will continue to develop for several hours, as evidenced by continued cell division and progression of eye differentiation. Development can be imaged through the glass from below using an inverted microscope, or from above using a water-immersion lens. There is the future prospect that eye disc culture methods could still improve further, perhaps using methods that have recently been described for wing disc culture [22, 23].
2.3 Dissection of larval eye imaginal discs for immunostaining
Unlike live imaging, fixed preparations represent only a snapshot of development. In the case of eye imaginal discs, however, the progression of the morphogenetic furrow and the repetitive, temporally staggered development of successive ommatidial columns provides a virtual time series in every disc and offers much more temporal information than for other tissues. For this and other reasons, antibody labeling to identify cell types and processes in fixed eye discs has been a mainstay of eye development studies.
Many published protocols describe the dissection and immunostaining of eye discs from late third instar larvae [24–28]. A brief compendium of antibody reagents useful for eye disc studies is also given below in section 2.5. The published protocols differ from the original, small-scale method developed by Tomlinson and Ready which facilitates the handling of one or a small number of imaginal discs, and also conserves reagents [4]. We give some details of this small-scale approach here.
2.3.1 Overview of the procedure
The general procedure is similar to that for any immunolabeling [29, 30]. It involves dissection, fixation, blocking, incubation in primary antibody, washing, incubation in secondary antibody, washing, and, for immunofluorescent studies, mounting prior to imaging.
2.3.2 Dissections
The dissection of eye-antennal imaginal discs from late third-instar larvae by pulling the mouthparts apart from the rest of the body using fine forceps has been described previously in both written and video protocols [24, 26–28]. The dissected material often initially includes extraneous tissue including brain and epidermis that can be easily be removed later during the blocking step rather than unnecessarily delaying fixation. For the purpose of handling individual discs, however, it is recommended that eye-antennal discs be left attached to the chitinous mouthparts, to which they are connected by nerves, throughout the antibody labeling and washing steps. The mouthparts, and any other remaining extraneous tissues, are removed during mounting onto slides.
2.3.3 Labeling and Transfers
Pairs of eye-antennal discs, each attached to the mouthparts, are transferred between solutions using a hook fashioned out of 0.005″ diameter tungsten wire (Ted Pella inc). Fix a 1–2 inch piece of wire in a holder. Using forceps, bend the tip of the wire to a ~90° angle. The bend should be as close to the tip as practical, so that the hooked portion is not much larger than an eye-imaginal disc. This tool can be used to lift eye-antennal disc pairs out of solution at the junction between the antennal disc and mouthparts. Transfers are thus effected without directly contacting eye disc tissue and with minimal transfer of solutions.
Labeling and washing steps are performed in 60-well plates. It is important that the wells are conical. We use MicroWell mini-trays (catalogue number 439225 from Nunc). Since we transfer imaginal discs between solutions, the 6×10 organization is convenient for processing 6 parallel samples through 10 solutions: 1° antibody, 3 washes, 2° antibody, 3 washes, 1 wash without detergent, 1 wash in mounting medium. The penultimate wash without detergent is important when detergent that are insoluble in glycerol are used. Each well may be filled with 13 μL of wash or antibody solution. Because of the small solution volumes, we avoid incubating more than ~5 eye-antennal disc pairs in each well.
2.3.4 Mounting
After clearing in mounting medium (we used 75% glycerol with 2% propyl gallate), transfer the discs to a drop of mounting medium on a clean slide and remove the mouthparts and any other unwanted tissue before sealing under a cover slip.
There are also variations of this procedure suitable for preparing immunostained whole mounts for embedding, sectioning and transmission electron microscopy.
2.4 Dissection of pupal eyes for immunostaining
2.4.1 Dissections
The dissection of the pupal retina has been described in several publications [24, 26, 28]. The methods work well between ~20-~50h after white puparium formation (at 25°C). The white puparium, at which animals will usually be staged, lasts ~2h. Once the desired stage is reached, immobilize pupae ventral side down on a petri dish lid using double-sided tape. Remove the hard pupal case from the posterior end until the pupa can be removed through the hole and placed in buffer.
In the published procedures a vertical slit is cut at the anterior of the transparent pupal cuticle and the brain and retina squeezed out and revealed when other material is washed away using a P20 micropipet. Alternatively, cut off the anterior half of the animal and gently wash out the contents until the brain and attached retinas are visible. Grab the brain to pull the retinas out of the pupa. It is often convenient for handling to keep the retinas attached to the brain during immunostaining.
2.4.2 Labeling and Transfer
Antibody labeling of pupal retinas has been described in several published protocols and videos [24, 26]. As with eye imaginal discs, these protocols can also be modified to reduce the scale. However, the thin pupal retinas are frequently damaged by repeated transfer between solutions, and we find it better to transfer them using a tungsten wire loop rather than a wire hook (use fine forceps to roll the wire into a loop at one end) Transfer in the buffer film that fills the loop avoids contact between the pupal retinas and the wire, and also avoids lifting the tissue in and out of the solution meniscus. We also use larger microwell plates, in which it is also an option to exchange wash solutions using drawn out pipets rather than transferring tissue between wells.
2.5 Antibodies for immunostaining eye development
Many, many antibodies have been used to detect proteins by immunostaining of larval eye imaginal discs and pupal retinas. Most need to be requested from their developers. A very basic collection of antibodies that would be useful in many different studies and that are available commercially includes:
Elav. Rat and Mouse monoclonal antibodies 7E8A10 and 9F8A9 (respectively) are available for this neuron-specific nuclear protein that is expressed in all the photoreceptor cells of the eye, and in the interommatidial bristle neuron that differentiates during the pupal stage (dshb.biology.uiowa.edu) [31].
Rough. A mouse monoclonal antibody 62C2A8 is available for this nuclear transcription factor that is stably expressed in photoreceptor cells R2,3,4,5 (dshb.biology.uiowa.edu) [32].
Cut. A mouse monoclonal antibody 2B10 is available for this nuclear transcription factor expressed in the non-neuronal cone cells (dshb.biology.uiowa.edu) [33].
Prospero. A mouse monoclonal antibody MR1A is available for this nuclear transcription factor expressed in the non-neuronal cone cells and the the R7 photoreceptor precursor (dshb.biology.uiowa.edu) [34, 35].
Eyes Absent. A mouse monoclonal antibody 10H6 is available for this nuclear transcription factor expressed in the eye primordium (dshb.biology.uiowa.edu) [36].
Dachshund. A mouse monoclonal antibody 2–3 is available for this nuclear transcription factor expressed in the eye primordium (dshb.biology.uiowa.edu) [37].
Crumbs. Mouse monoclonal antibody Cq4 is available for this apical transmembrane protein that outlines cells in the eye imaginal disc and labels the rhabdomere stalk in the mature photoreceptor cells (dshb.biology.uiowa.edu) [38].
Armadillo (beta-catenin). Mouse monoclonal antibody N27A1 is available for this adherens junction associated protein that outlines cells in the eye imaginal disc (dshb.biology.uiowa.edu) [39].
Discs Large. Mouse monoclonal antibody 4F3 is available for this apical membrane protein that outlines imaginal disc cells (dshb.biology.uiowa.edu) [40].
Cyclin-B. Mouse monoclonal antibody F2F4 is available that specifically labels cells progressing through S- and G2-phases of the cell cycle [41].
phospho-Histone H3 (Ser10). Rabbit polyclonal antibody is available that specifically stains chromatin of mitotic cells (Cell Signaling Technology #9701)[42].
Cleaved Drosophila Dcp-1. Rabbit polyclonal antibody is available that specifically stains apoptotic Drosophila cells (Cell Signaling Technology #9578).
In addition to these and many other endogenous proteins, the beta-galactosidase and Green Fluorescent Protein are expressed in many transgenes and enhancer traps. Mouse monoclonal antibody 40-1 (dshb.biology.uiowa.edu) and rabbit polyclonal antibodies (Cappell) are available to detect beta-galactosidase in tissue. Mouse, Rat and Rabbit antibodies are available to detect GFP in tissue (Invitrogen).
2.6 Fixatives and wash buffers for immunostaining eye discs
Many variations in fixation, preadsorbtion, antibody incubation and wash buffers are reported in the literature [24–28] [29, 30]. In many cases these variations are inconsequential. By contrast, some antibody:antigen interactions are quite particular and protocol details will have significant impact. Therefore, it will sometimes be worthwhile to follow published protocols precisely or to optimize immunostaining in your own hands.
Two fixation methods in common use:
4% formaldehyde in 0.1M PIPES (pH 7.0) 2mM MgSO4 1mM EGTA, fix eye discs for ~30 min at room temperature;
or
2% paraformaldehyde, 10mM NaIO4 75mM lysine 37mM NaPO4 (pH 7.2), also known as PLP. PLP is a milder protein fix that also crosslinks carbohydrates, and better preserved membrane structures. Eye discs are usually fixed in PLP for 45 min on ice.
Two antibody and wash buffers in commonly use:
0.1M NaPO4 (pH 7.2), 0.3% sodium deoxycholate 0.3% Triton X-100;
or
0.1M NaPO4 (pH 7.2), 0.1% saponin, 5% normal serum. Saponin is a mild, non-ionic detergent that will preferentially permeabilize the plasma membrane, especially if used at still lower concentrations.
2.7 In situ hybridization to Drosophila eye imaginal discs
2.7.1 General aspects
The use of non-radioactive, digoxigenin (DIG)-labeled probes to hybridize to target RNAs was pioneered by Tautz and Pfeifle [43]. This approach, using histochemical signal detection with Alkaline Phosphatase-conjugated secondary antibodies, remains widely used. See Cornell et al (1999) for an updated protocol [44].
The advantages of fluorescent in situ hybridization (FISH) include simultaneous detection of multiple probes and more precise spatial localization than is permitted by the diffusible histochemical products. We will briefly outline RNA probe preparation, tissue preparation, hybridization and signal detection and some recent modifications that have been described.
2.7.2 RNA probe preparation
Most reliable probes are around 400–600 bp in length. One needs to make sure probes are unique to the gene of interest by BLAST (http://flybase.org/blast/). Probes spanning multiple exons are thought to reduce potential hybridization to genomic DNA or pre-mRNA. Locked Nucleic Acid (LNA) probes may be useful in certain circumstances [45].
The DNA template for RNA probe synthesis by in vitro transcription can be a linearized plasmid from a vector that has phage transcriptional promoters (e.g. T3, T7, SP6) [46], or the template can be a PCR product where the promoter sequences were included in the primers [47].
Kits for in vitro transcription with diverse probe labels are available commercially, such as DIG, biotin and fluorescein (Roche) and dinitrophenyl (PerkinElmer). These can be used to distinguish multiple transcripts in parallel.
2.7.3 Tissue preparation
Tissue can be dissected as discussed above (2.3.2). We generally use formaldehyde fixative prepared freshly from paraformaldehyde powder.
Fixed tissue can be stored in methanol at −20°C after fixation and before use (up to weeks). Rehydration must be performed through a graded methanol series and a short post-fixation might be necessary. Some workers feel that methanol storage >1 week improves results
Proteinase K was traditionally used to increase transcript accessibility in tissue at the expense of tissue morphology. Many workers, however, now omit this step for eye disc in situ hybridization.
2.7.4 Hybridization
Tissue should be pre-hybridized in probe-free hybridization solution to block non-specific binding. RNA probes should be denatured at 80–90°C for 10 min before use. Hybridization is typically performed overnight.
2.7.5 Signal detection
Primary antibodies against labeled probes are detected using fluorophore-conjugated secondary antibodies. If double labeling, one needs to be concerned about the cross-reactivity of antibodies.
Tyramide signal amplification (PerkinElmer) is commonly used to amplify FISH signal. Instead of fluorophore-conjugated secondary antibodies, this method introduces a horseradish peroxidase (HRP)-conjugated secondary antibody, which can catalyze and deposit fluorophore-conjugated tyramide. A variety of fluorophore-labeled TSA reagents make sequential TSA reactions feasible in Drosophila [48]and other organisms [49].
2.7.6 Simultaneous detection of RNA and protein
One advantage of using Drosophila is the ease of mosaic analysis. It is useful and informative therefore to combine FISH with simultaneous detection of clonal genotypes that are typically marked with arm-LacZ or ubi-GFP transgenes. There are also other circumstances where simultaneous detection of RNA and protein is useful. The challenge is to find a balance between FISH and immunostaining conditions that produces a decent result with each.
It is usual to omit the ProK treatment since it can damage the epitopes. We have had success in our lab by adding primary antibody against GFP (which marks our clones) to the HRP-conjugated antibody solution used to detect the hybridized RNA probe. Secondary antibody incubation to label the GFP signal fluorescently and the in situ signal with HRP is then followed by separate TSA amplification for the FISH signal. Published protocols are similar with some variations, for example Reynolds-Kennealy and Mlodzik (2005) used further series amplification of FISH signal [50], while VanZomeren-Dohm et al (2008) detected unamplified FISH signal [51]. Nagaso et al. (2001) suggested using acetone to make membranes accessible to RNA probes and antibodies while maintain tissue integrity and antigenicity [52].
An alternative approach, one that emphasizes protein detection, is to complete the entire antibody staining procedure for GFP or beta-galactosidase before beginning hybridization. A second fixation is performed after antibody staining to preserve this signal through the hybridization process [53, 54].
2.8 Dissociating eye imaginal discs
Dissociating eye imaginal discs is useful for single cell imaging, for FACS sorting to assess cell cycle or isolate subpopulations for RNA preparation or other studies. A general procedure for dissociating imaginal discs using trypsin has been published [55]. The early differentiation of neurons in the eye disc and the highly ordered structure of the retina require some modifications.
Stage animals at the white prepupa and dissect eye discs for dissociation within ~1h. Remove eye-antennal discs from the prepupa as described for late third-instar larvae (section 2.3.2). Remove all extraneous tissue such as mouthparts and optic lobes of the brain before dissociation. If the goal is to isolate developing photoreceptor neurons then it may be advisable to remove the antennal disc, since this also contains neurons.
Disc dissociation is facilitated if the peripodial membrane is first disrupted. The thin peripodial membrane overlies the apical surface of the eye and antennal disc epithelium proper. The peripodial membrane is best visualized using oblique illumination from fibre optic light sources. The dissection is facilitated in a Sylgard dissecting dish. Hold the antennal disc with fine forceps so that the eye disc is held edge-on to the substrate. Use a fine scalpel (#11 blade) or sharp tungsten needle to pierce the peripodial membrane and peel it from the disc proper. These manipulations will crush the antennal disc, which should be discarded.
Transfer discs into a glass well containing the Trypsin dissociation solution. Pool discs during a 45 min time window. Use a P1000 pipetman, with the pipet tip trimmed to increase the diameter, to gently transfer 200 μL of solution and discs to a glass test tube. Place the tube at a 45°C angle on a mechanical shaker and agitate gently. Dissociation should be calibrated according to local conditions, but dissociation times of ~90–120 min are anticipated.
2.9 Scanning Electron Microscopy of Adult Eyes
SEM is the best way to record the external structure of the adult eye. It offers high resolution and, compared to light microscopy, far superior depth of field. The procedures have been summarized by Wolff [56]. In many instances, preparation and imaging will involve contributions from an institutional electron microscopy facility and the protocol to be used locally will be determined in consultation with them. The degree of tissue fixation that is necessary may depend on whether critical point drying is to be performed and on the frequency of tissue collapse that can be tolerated ie whether the specimens can be replaced.
We use this procedure.
Fixation. Fix the whole animals in 2.5% glutaraldehyde, 0.1 M sodium Cacodylate, 5 mM MgCl2 (pH 7.4) for 2h, and then rinse in H2O. Note this step needs to be processed in the fume hood. The purpose of this fixation step is to improve the yield of good specimens, but it is possible to obtain satisfactory SEM results without this step.
Incubate the whole animal through a graded ethanol series. Place 10 flies into 5 ml of 25% ethanol and incubate for 12–24 hrs at room temperature. Then transfer and incubate the flies for 12–24 hrs at room temperature through 50% and 75% ethanol once, and twice in 100% ethanol. The exterior eye morphology is stable for up to one month in the second 100% ethanol solution.
The following steps are performed by the Analytical Imaging Facility at our institution (Albert Einstein College of Medicine). Critical point drying is performed to exchange the ethanol for liquid carbon dioxide using a Tousimis Samdri 795 Critical Point Drier (Rockville MD). Specimens are sputter coated with chromium in a Quorum EMS 150T ES (Quorum Technologies Ltd). The samples are now ready to be imaged using a Zeiss Supra Field Emission Scanning Electron Microscope (Carl Zeiss Microscopy, LLC). It is important that the specimens have been mounted in an orientation that facilitates imaging the eye, and that the eyes are free of dirt and the mounting adhesive.
2.10 Photographing the adult eye
Although SEM is the best way to record adult eye structure, it does not record color. SEM is unsuitable for recording, for example, studies of pigmentation mechanisms, or that make use of differently-colored eye cell populations to compare clonal growth rates. To immobilize living flies for photomicrography, anesthetize them with ether and mount them on slides coated with double-sided tape. Alternatively, if surviving flies are not required, store flies frozen at −20°C, then image the frozen flies later.
Adult eyes can be photographed using either compound or dissecting microscopes, so long as the magnification is appropriate and reflected light available. The major challenge with photographing the eye is depth of field. Since the eye surface is curved, very little will usually be in any given focal plane. Depth of focus is determined, as in other photography, by focal length and aperture of the lens. Since few microscopes have variable aperture lenses, focus stacking may be required. Acquire a series of images focused on different positions in the z-axis ie from the surface of the eye to the bristles of the head cuticle that surround the eye margin. Import these images into Adobe Photoshop as separate layers and use the Edit>Auto-Align Layers and then Edit>Auto-Blend Layers commands to generate a composite image that maximizes focus. Alternatively, specialist software such as Helicon Remote and Helicon Focus (HeliconSoft Inc) can direct both the acquisition and blending of image stacks.
2.11 Sectioning the adult retina for light microscopic studies
The internal anatomy of the adult retina is most commonly studied by thick sectioning of plastic-embedded heads [57, 58]. A video protocol is available [59]. The head is removed from the body. Some portion of the cuticle must also be removed to facilitate fixation; usually one eye is cut off and discarded to allow fixative into the rest of the head and contralateral eye. The procedure is similar to that used for fixing, sectioning and embedding for EM purposes except that softer resin mixes are appropriate for thick sections. Sections between 1 micron and 4 microns can be useful depending on the intended purpose, for example thicker sections may be chosen if pigment content is to be assessed. Be sure to avoid exposure to gluaraldehyde and osmium tetroxide by using a fume hood to handle these fixatives.
2.12 Immunostaining the adult retina
2.12.1 Immunostaining sections of the adult retina
Cryosectioning is used if sections are to be immunostained [58, 60]. Dissected heads are fixed in 3% formaldehyde in PBS on ice for 60–90 min. After rinsing, heads are transferred to 12% sucrose in PBS and bissected. Tissue is infiltrated with 12% sucrose in PBS for 4–12h at 4°C. Tissue is then permeated in OCT Tissue Tek Compound for cryosectioning. Sections are dried on slides and postfixed in 0.5% formaldehyde prior to immunostaining.
2.12.1 Immunostaining adult retina wholemounts
Alternatively, the adult retina can be immunostained as a wholemount. After removal, adult heads are further dissected in buffer to remove the retina. After fixation, the lamina is removed and an overnight wash in PBT extracts autofluorescent pigments from the photoreceptors. A video protocol describing this procedure is available [61].
2.13 Direct live imaging of retina structure
There are other methods for studying the internal retinal structure of adult flies. Pichaud & Desplan [62] describe how to observe photoreceptor cells in living flies by using water immersion objectives to visualize visible-light absorption, or by using epifluorescence imaging with various wavelengths to image either Rhodopsin 1 or its UV-sensitive accessory pigment in photoreceptor cells. Epifluorescence can also be used to reveal photoreceptor-expressed GFP in living flies.
2.14 Genetic Tools for Drosophila eye studies
Because a number of genetic tools have been developed for the Drosophila eye, and since this organ is largely dispensable for survival and fertility, the eye is commonly used for studies that in principle could also be performed in other organs.
2.14.1 Eye-specific expression and knockdown using the GAL4/UAS system
Gene expression and knockdown in developing eye tissues can be useful for its own sake but also is frequently employed as a basis for genetic modifier screens.
The GAL4/UAS system is based on the yeast GAL4 protein which activates transcription of the target genes by binding to the upstream activating sequence (UAS). In Drosophila, these two components are carried in separate flies and combined by breeding [63]. The GAL4 drivers provide tissue-specific GAL4 protein expression, while the transgene lines carrying the UAS region next to the coding region for the gene of interest.
Gal4 lines that are commonly used for eye expression are eyeless (ey)-GAL4(expression in growing eye disc cells ahead of the morphogenetic furrow)[64], (GMR)-GAL4 (expressed in all eye disc cells posterior to the morphogenetic furrow)[65], and sevenless (sev)-GAL4 (expressed in a subset of cells differentiating behind the morphogenetic furrow)[63].
Many UAS lines have been developed for the expression of individual genes and are available from their developers. In addition, collections of transposon insertions exist that introduce UAS elements at diverse positions in the genome where they can control endogenous genes [66–68].
Other collections of UAS lines are designed to express RNA hairpins that initiate gene-specific RNA silencing. Information on such insertions is stored at Flybase. Many of these lines are available from their developers in Vienna, Harvard, and Mishima (stockcenter.vdrc.at/control/main; www.flyrnai.org/TRiP-HOME.html; www.shigen.nig.ac.jp/fly/nigfly/about/aboutRnai.jsp).
2.14.2 Conditional mutation in the eye
Homozygous mutant cells are routinely generated in Drosophila using the site-specific FRT/FLP recombination system from yeast. FRT sites integrated at the base of chromosome arms permit mitotic recombination by chromosome arm exchange [69–71]. When combined with the Gal4/UAS and Gal80 genes in the MARCM system, transgenic gene expression can be targeted to the recombinant clones [72].
The ey-FLP transgenes, active in the developing eye disc, typically convert the head of heterozygous animals to an equal mixture of the two reciprocal homozygous genotypes [73]. This approach can easily be used as an assay for relative growth rate, by comparing the relative contributions of the two genotypes to the adult [74, 75]. When one of the homozygous genotypes is cell-autonomously lethal, for example through mutation of an essential cell cycle gene or component of the ribosome, adult heads that are almost entirely composed of the reciprocal homozygote result [73]. This facilitates study of the adult head phenotype of embryonic lethal mutations, for example [76]. Note, however, that if both homozygotes are cell-autonomously lethal, or have a severe phenotype, the mosaic animal will have no functional head and early lethality is to be expected. One consequence is that strains containing ey-Flp and FRT-linked mutations may not be appropriate for genetic complementation tests.
The GMR-Flp transgene, active posterior to the morphogenetic furrow, can only recombine cells that divide in the Second Mitotic Wave or interommatidial bristle organs, and will usually generate single-cell clones [77]. This has been useful for generating single photoreceptor neurons of particular genotypes, for example in assessing axon targeting phenotypes [78, 79].
2.14.3 Genetic screens in the eye
These methods for manipulating eye genotypes have been applied in many genetic screens. Although basic screens to identify genes modifying growth or required for eye differentiation have already been done [74, 75, 80], the genetic basis for novel processes of interest can be explored. For example, mosaic mutant clones, RNA knockdowns, or targeted gene expression, can be examined for effects on the choice of specific rhodopsin expressed by retinal photoreceptors [81].
2.14.4 Genetic Modifier screen in the eye
Particular mention must be made about genetic modifier screens. These rely on the empirical observation that, when development is perturbed in a dose-sensitive manner, the resulting phenotype commonly becomes sensitive to the heterozygous loss of other loci functioning in the same pathway, even though these loci are not haploinsufficient in a wild type background. Although first exploited in studies of sensory bristle development [82], this approach leapt to prominence through its successful use to delineate the main receptor tyrosine kinase and Ras signaling pathways [5–7]. Now and in the future, genetic modifier screens are likely to be useful not only for the elucidation of normal developmental pathways, but also to determine the pathogenic mechanisms of loci associated with human disease, as for example in the investigation of pathogenic mechanisms of ataxin-3 toxicity [83].
In one approach, novel mutagenesis is undertaken and mutated chromosomes screened for modification of the phenotype of interest, often obtained using one of the conditional mutation, knockdown, or misexpression approaches described in this chapter. Once novel modifiers are isolated, they must be mapped and identified. An example would be the identification of Su(H) as a signal transducer of Notch signaling [8].
In a second approach, one or more of several large collections of deletion chromosomes is crossed into the sensitized genotype of interest, and chromosome intervals that contain modifiers identified [84, 85]. This approach is faster initially, although further work must usually be done to identify individual modifier loci within specific intervals, and to date deficiency collections are not complete although they do cover ~80% of known genes. It is important to use a deficiency collection of homogenous genetic background, usually either the DrosDel or Exelixis collections. An example would be the identification of genes mediating myc-induced cell growth [86].
Acknowledgments
We thank Andreas Jenny fro providing Figure 1B and John Fullard for reading the manuscript. Supported by grants from the NIH (GM047892 and EY021614), by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, and by a 2012 Established Investigator Award from Research to Prevent Blindness to NEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- 2.Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Developmental Biology. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence PA, Green SM. Cell lineage in the developing retina of Drosophila. Developmental Biology. 1979;71:142–152. doi: 10.1016/0012-1606(79)90088-5. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson A, Ready DF. Neuronal differentiation in the Drosophila ommatidium. Developmental Biology. 1987;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 5.Karim FD, Chang HC, Therrien M, Wassarman DA, Laverty T, Rubin GM. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics. 1996;143:315–329. doi: 10.1093/genetics/143.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon MA, Bowtell DDL, Dodson GS, Laverty TR, Rubin GM. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 7.Simon MA, Dodson GS, Rubin GM. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993;73:169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- 8.Fortini ME, Artavanis-Tsakonas S. The Suppressor of Hairless protein participates in Notch signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 9.Verheyen EM, Purcell KJ, Fortini ME, Artavanis-Tsakonas S. Analysis of dominant enhancers and suppressors of activated Notch in Drosophila. Genetics. 1996;144:1127–1141. doi: 10.1093/genetics/144.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong X, Zavitz KH, Thomas BJ, Lin M, Campbell S, Zipursky SL. Control of G1 in the developing Drosophila eye: rca1 regulates Cyclin A. Genes and Development. 1997;11:94–105. doi: 10.1101/gad.11.1.94. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SM. Imaginal disc development. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. II. CSHL Press; 1993. pp. 747–841. [Google Scholar]
- 12.Green P, Hartenstein AY, Hartenstein V. The embryonic development of the Drosophila visual system. Cell Tissue Res. 1993;273:583–598. doi: 10.1007/BF00333712. [DOI] [PubMed] [Google Scholar]
- 13.Ashburner M, Golic KG, Hawley RS. Drosophila A Laboratory Handbook. 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- 14.Baker NE. Notch and the patterning of ommatidial founder cells in the developing Drosophila eye. Results and Problems in Cell Differentiation. 2002;37:35–58. doi: 10.1007/978-3-540-45398-7_4. [DOI] [PubMed] [Google Scholar]
- 15.Treisman JE. Retinal differentiation in Drosophila. Wiley Interdiscip Rev Dev Biol. 2013;2:545–557. doi: 10.1002/wdev.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaraj R, Canon J, Banerjee U. Cell fate specification in the Drosophila eye. Results and Problems in Cell Differentiation. 2002;37:73–88. doi: 10.1007/978-3-540-45398-7_6. [DOI] [PubMed] [Google Scholar]
- 17.Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson M, Saint R. A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol. 1999;18:457–462. doi: 10.1089/104454999315178. [DOI] [PubMed] [Google Scholar]
- 19.Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139:3432–3441. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Ohsawa S, Sugimura K, Takino K, Igaki T. Imaging cell competition in Drosophila imaginal discs. Methods Enzymol. 2012;506:407–413. doi: 10.1016/B978-0-12-391856-7.00044-5. [DOI] [PubMed] [Google Scholar]
- 22.Aldaz S, Escudero LM, Freeman M. Live imaging of Drosophila imaginal disc development. Proc Natl Acad Sci U S A. 2010;107:14217–14222. doi: 10.1073/pnas.1008623107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zartman J, Restrepo S, Basler K. A high-throughput template for optimizing Drosophila organ culture with response-surface methods. Development. 2013;140:667–674. doi: 10.1242/dev.088872. [DOI] [PubMed] [Google Scholar]
- 24.Wolff T. Histological techniques for the Drosophila eye Part 1: Larva and Pupa. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. pp. 201–227. [Google Scholar]
- 25.Firth LC, Li W, Zhang H, Baker NE. Analyses of RAS regulation of eye development in Drosophila melanogaster. Methods Enzymol. 2006;407:711–721. doi: 10.1016/S0076-6879(05)07056-4. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao HY, Johnston RJ, Jukam D, Vasiliauskas D, Desplan C, Rister J. Dissection and immunohistochemistry of larval, pupal and adult Drosophila retinas. J Vis Exp. 2012:e4347. doi: 10.3791/4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purves DC, Brachmann C. Dissection of imaginal discs from 3rd instar Drosophila larvae. J Vis Exp. 2007:140. doi: 10.3791/140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff T. Dissection techniques for pupal and larval Drosophila eyes. CSH Protoc. 2007;2007 doi: 10.1101/pdb.prot4715. pdbprot4715. [DOI] [PubMed] [Google Scholar]
- 29.Klein T. Immunolabeling of imaginal discs. Methods Mol Biol. 2008;420:253–263. doi: 10.1007/978-1-59745-583-1_15. [DOI] [PubMed] [Google Scholar]
- 30.Blair SS. Triple-label fluorescent antibody staining of imaginal discs in Drosophila. CSH Protoc. 2007;2007 doi: 10.1101/pdb.prot4796. pdbprot4796. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- 32.Kimmel BE, Heberlein U, Rubin GM. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes and Development. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- 33.Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 1990;4:1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 34.Spana EP, Doe CQ. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–3195. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- 35.Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW. Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev. 1996;10:2167–2178. doi: 10.1101/gad.10.17.2167. [DOI] [PubMed] [Google Scholar]
- 36.Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 37.Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 38.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 39.Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- 40.Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclina A and B during the G2-M transition. European Molecular Biology Organization Journal. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Nooij JC, Hariharan IK. Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science. 1995;270:983–985. doi: 10.1126/science.270.5238.983. [DOI] [PubMed] [Google Scholar]
- 43.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 44.Cornell M, Evans DA, Mann R, Fostier M, Flasza M, Monthatong M, Artavanis-Tsakonas S, Baron M. The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics. 1999;152:567–576. doi: 10.1093/genetics/152.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomsen R, Nielsen PS, Jensen TH. Dramatically improved RNA in situ hybridization signals using LNA-modified probes. RNA. 2005;11:1745–1748. doi: 10.1261/rna.2139705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lecuyer E, Parthasarathy N, Krause HM. Fluorescent in situ hybridization protocols in Drosophila embryos and tissues. Methods Mol Biol. 2008;420:289–302. doi: 10.1007/978-1-59745-583-1_18. [DOI] [PubMed] [Google Scholar]
- 47.Firth LC, Baker NE. Spitz from the retina regulates genes transcribed in the second mitotic wave, peripodial epithelium, glia and plasmatocytes of the Drosophila eye imaginal disc. Dev Biol. 2007;307:521–538. doi: 10.1016/j.ydbio.2007.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkie GS, Davis I. Visualizing mRNA by in situ hybridization using ‘high resolution’ and sensitive tyramide signal amplification. Technical Tips Online. 1998;3:94–97. [Google Scholar]
- 49.Brend T, Holley SA. Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J Vis Exp. 2009 doi: 10.3791/1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Developmental Biology. 2005;285:38–48. doi: 10.1016/j.ydbio.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 51.VanZomeren-Dohm A, Flannery E, Duman-Scheel M. Whole-mount in situ hybridization detection of mRNA in GFP-marked Drosophila imaginal disc mosaic clones. Fly (Austin) 2008;2:323–325. doi: 10.4161/fly.7230. [DOI] [PubMed] [Google Scholar]
- 52.Nagaso H, Murata T, Day N, Yokoyama KK. Simultaneous detection of RNA and protein by in situ hybridization and immunological staining. J Histochem Cytochem. 2001;49:1177–1182. doi: 10.1177/002215540104900911. [DOI] [PubMed] [Google Scholar]
- 53.Goto S, Hayashi S. Cell migration within the embryonic limb primordium of Drosophila as revealed by a novel fluorescence method to visualize mRNA and protein. Development Genes and Evolution. 1997;207:194–198. doi: 10.1007/s004270050107. [DOI] [PubMed] [Google Scholar]
- 54.Toledano H, D’Alterio C, Loza-Coll M, Jones DL. Dual fluorescence detection of protein and RNA in Drosophila tissues. Nat Protoc. 2012;7:1808–1817. doi: 10.1038/nprot.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Cruz AF, Edgar BA. Flow cytometric analysis of Drosophila cells. Methods Mol Biol. 2008;420:373–389. doi: 10.1007/978-1-59745-583-1_24. [DOI] [PubMed] [Google Scholar]
- 56.Wolff T. Preparation of Drosophila eye specimens for scanning electron microscopy. Cold Spring Harb Protoc. 2011;2011:1383–1385. doi: 10.1101/pdb.prot066506. [DOI] [PubMed] [Google Scholar]
- 57.Gaengel K, Mlodzik M. Microscopic analysis of the adult Drosophila retina using semithin plastic sections. Methods Mol Biol. 2008;420:277–287. doi: 10.1007/978-1-59745-583-1_17. [DOI] [PubMed] [Google Scholar]
- 58.Wolff T. Histological techniques for the Drosophila eye Part II: Adult. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 59.Jenny A. Preparation of adult Drosophila eyes for thin sectioning and microscopic analysis. J Vis Exp. 2011 doi: 10.3791/2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolff T. Cryosectioning and immunocytochemistry of adult Drosophila eye sections. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5370. pdbprot5370. [DOI] [PubMed] [Google Scholar]
- 61.Williamson WR, Hiesinger PR. Preparation of developing and adult Drosophila brains and retinae for live imaging. J Vis Exp. 2010 doi: 10.3791/1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pichaud F, Desplan C. A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development. 2001;128:815–826. doi: 10.1242/dev.128.6.815. [DOI] [PubMed] [Google Scholar]
- 63.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 64.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 65.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 66.Rorth P, Szabo K, Bailey A, Laverty T, Rehm J, Rubin GM, Weigmann K, Milan M, Benes V, Ansorge W, et al. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 67.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toba G, Ohsako T, Miyata N, Ohtsuka T, Seong KH, Aigaki T. The gene search system. A method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics. 1999;151:725–737. doi: 10.1093/genetics/151.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- 70.Xu T, Rubin GM. Analysis of genetic mosaics in the developing and adult Drosophila tissues. Development. 1993;117:1223–1236. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 71.Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee T, Luo L. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 73.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 74.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosus complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 75.Moberg KH, Bell DW, Wahrer DCR, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and id mutated in human cancer cell lines. Nature. 2001;413:311–316. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharya A, Baker NE. The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye. Dev Biol. 2009;327:288–300. doi: 10.1016/j.ydbio.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 78.Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 79.Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 80.Janody F, Lee JD, Jahren N, Hazelett DJ, Benlali A, Miura GI, Draskovic I, Treisman JE. A mosaic genetic screen reveals distinct roles for trithorax and polycomb group genes in Drosophila eye development. Genetics. 2004;166:187–200. doi: 10.1534/genetics.166.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 82.Botas J, Moscoso del Prado J, Garcia-Bellido A. Gene-dose titration analysis in the search of trans-regulatory genes in Drosophila. EMBO J. 1982;1:307–310. doi: 10.1002/j.1460-2075.1982.tb01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bilen J, Bonini NM. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet. 2007;3:1950–1964. doi: 10.1371/journal.pgen.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryder E, Ashburner M, Bautista-Llacer R, Drummond J, Webster J, Johnson G, Morley T, Chan YS, Blows F, Coulson D, et al. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics. 2007;177:615–629. doi: 10.1534/genetics.107.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 86.Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]


