Figure 3.
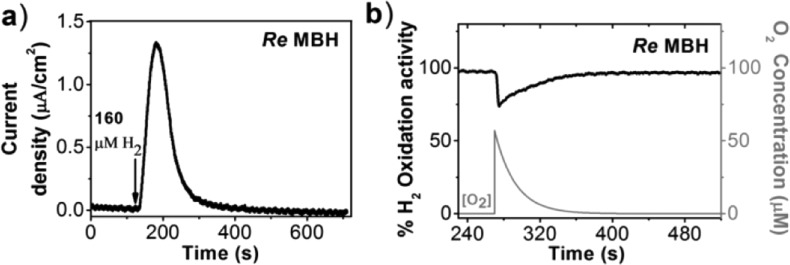
(a) Chronoamperogram showing the evolution of the MBH activity after the injection of a H2-saturated aliquot of buffer into the cell solution flushed with N2 (+0.497 V vs SHE; 30 °C, pH 7.4). (b) Chronoamperometry of the MBH (+0.397 V vs SHE, 100% H2, pH 7.4, 30 °C). The current is used to determine the hydrogen oxidation activity, which is normalized to 100% at the start of the experiment. An aliquot (one-fourth volume of the final cell volume) of air-saturated buffer was inserted into the electrochemical cell at 270 s. The exponential decay of the O2 concentration was plotted according to the equation: C(t) = C(0) exp(−t/τ), C is concentration, t is time), and τ is the time constant for exponential gas removal and was determined to be 22 s under these conditions (see Figure S2).
