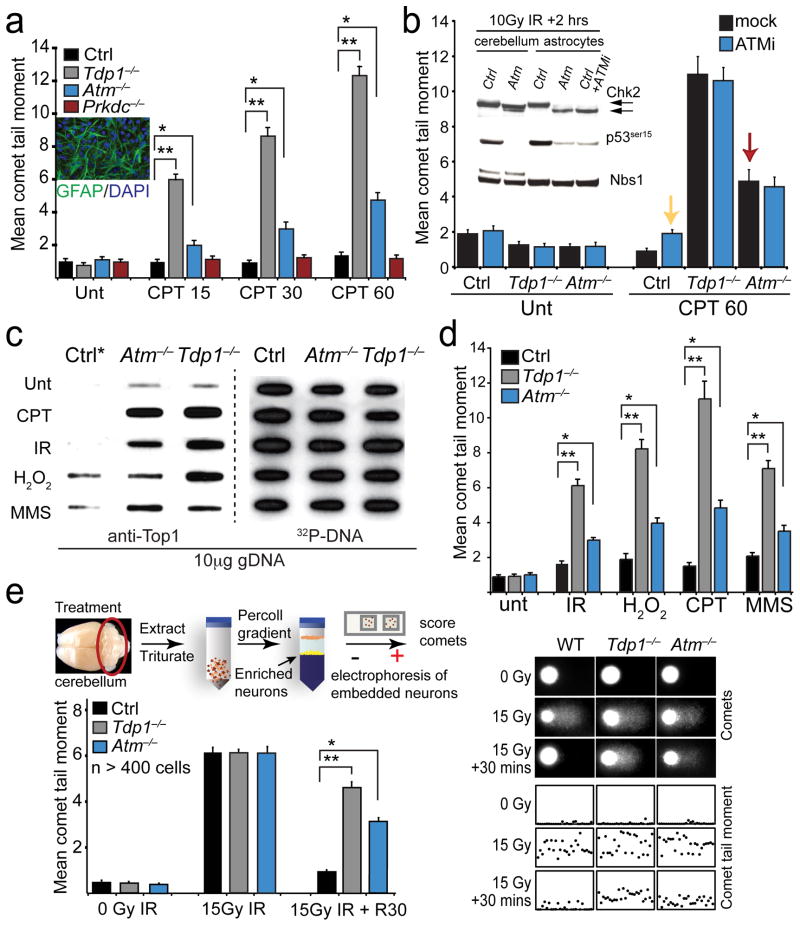
Fig. 2. Atm is required for the normal response to the topoisomerase 1 poison, camptothecin.
a. The repair kinetics of quiescent astrocytes following treatment with the Topoisomerase-1 poison camptothecin (CPT) at the indicated time points is shown. Although Top1-induced DNA breaks are repaired more slowly in Atm−/− astrocytes, deficiency in the related kinase, DNA-dependent protein kinase, catalytic subunit (Prkdc−/−) results in comparable DNA single-strand break repair rates as wild-type (Ctrl) astrocytes. Inset panel shows quiescent GFAP-positive Atm−/− astrocytes. b. Inhibiting Atm kinase activity fails to recapitulate the repair defect observed in Atm−/− cells after CPT. Western blot analysis (inset) of irradiated wild type (Ctrl) astrocytes co-treated with 10 μM ATM inhibitor KU55933 (ATMi) and radiation confirms Atm inhibition by defective DNA damage-induced Chk2 modification (black arrows) and p53 phosphorylation in Atm−/− cerebella and astrocytes. NBS1 was used as a loading control. Comet analysis (bar graph) indicates Atm−/− astrocytes (red arrow) accumulate significantly more CPT-induced DNA damage than ATMi-treated ctrl astrocytes (yellow arrow), indicating ATM kinase-independent repair of Top1-DNA lesions. Full-length Western blots are presented in Supplementary Figure 11. c. ICE analysis of quiescent primary murine astrocytes following treatment with DNA damaging agents can result in accumulation of Top1cc. Treatment conditions were; 14 μm CPT for 60 mins at 37°C; 20Gy IR followed by 60 mins recovery at 37°C; 150 μm H2O2 for 5 mins at 4°C followed by 60 mins recovery at 37°C; 0.20 mg/ml MMS for 10 mins at 37°C. Top1cc were identified by blotting genomic DNA with anti-Top1 and gDNA levels were assessed by re-probing with 32P-labelled mouse ES cell genomic DNA (32P-DNA). d. Alkaline comet analysis of quiescent Atm−/− astrocytes show defective DNA single-strand break repair after treatments listed for ‘c’. For each in vitro comet assay, 100 cells/comet corresponding to each genotype and treatment were analyzed and experiments were performed in triplicate (total of n=300 cells/genotype/treatment). e. In vivo comet analysis comparing relative DNA strand break repair rates amongst ctrl, Atm−/− and Tdp1−/− cerebellar granule cell neurons following ionizing radiation (15 Gy) and a 30 min recovery. Bar graphs represent mean comet tail moments from experiments that were repeated in duplicate (2 mice/treatment) with cells isolated from each cerebella also measured in duplicate (total n=400 independent comet tail moments measured per line/treatment); error bars represent standard error of means (S.E.M.). Scatterplots indicate representative cellular comet tail moments from each corresponding cell/treatment type. For all graphs */** denotes p-values < 0.0001.